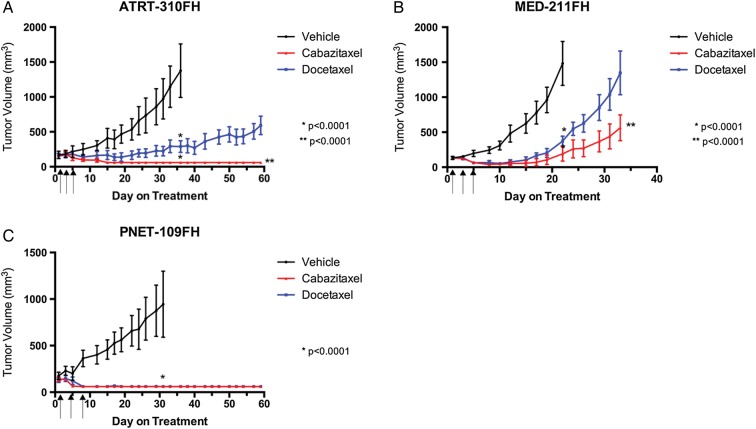
Fig. 2.
Antitumor activity of cabazitaxel and docetaxel in pediatric patient-derived flank xenografts. Patient-derived models of ATRT, medulloblastoma, and CNS-PNET were transplanted as subcutaneous flank xenografts into female nude mice. Treatment groups were randomly assigned when tumors reached 150–300 mm3. Cabazitaxel or docetaxel were administered on days 1, 3, and 5 by i.p. injection at 15 mg/kg. Data are reported as mean tumor volume ± SEM. Statistical analysis of tumor growth was performed comparing tumor volume of the vehicle with the cabazitaxel and docetaxel treatment groups at the time the vehicle group was removed due to tumor size. Cabazitaxel and docetaxel treatment groups were allowed to continue for 60 days or until the tumor volume limit was reached. Statistical analysis comparing cabazitaxel to docetaxel was performed at the termination of the study. (A) ATRT-310FH; (B) MED-211FH; (C) PNET-109FH.