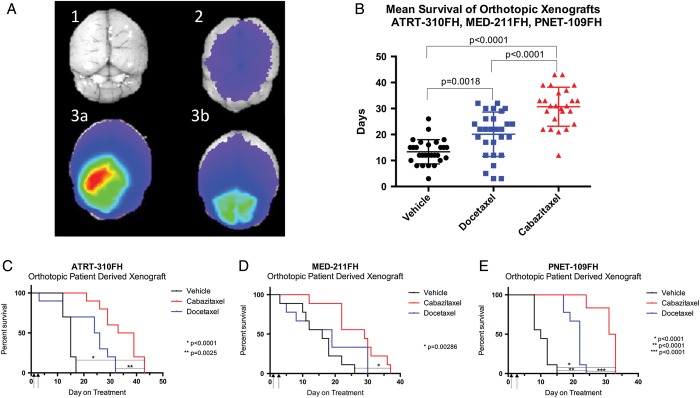
Fig. 4.
Comparison of antitumor activity of cabazitaxel and docetaxel in orthotopic pediatric patient-derived xenografts. Patient-derived models of ATRT, medulloblastoma, and CNS-PNET were transplanted as orthotopic xenografts into the cerebrum (ATRT-310FH and PNET-109FH) or the cerebellum (MED-211FH) of male or female NOD:scid gamma mice. Either cabazitaxel or docetaxel was administered on days 1 and 3 by i.p. injection at 15 mg/kg. (A) To determine readiness of orthotopic xenografts for study enrollment, tumor burden was assessed in 2 randomly chosen mice. Mice received an i.v. injection of chlorotoxin-Cy5.5, and brains were imaged ex vivo in the near-infrared spectrum 3 hours after injection. Positive signal, indicating significant, but not symptomatic, tumor burden triggered study initiation. (A1) Nontumor-bearing, noninjected negative control brain. (A2) Nontumor-bearing, CTX-Cy5.5-injected negative control brain. (A3A and A3B) Representative, Med-211FH tumor-bearing, CTX-Cy5.5 injected brains ex vivo. (B) Combined mean survival of the ATRT-310FH, MED-211FH, and PNET-109FH orthotopic models. Mouse survival by treatment group in individual tumor models. (C) ATRT-310FH; (D) MED-211FH; and (E) PNET-109FH.