Abstract
Background
While the impact of cranial radiation on white matter following treatment for pediatric brain tumor has been the focus of many recent studies, the effect of treatment in the absence of radiation has received little attention. The relations between white matter and cognitive outcome have not been explored in patients who have undergone radiation-free treatment. As most patients treated without cranial radiation survive long after their diagnosis, it is critical to identify factors that may impact structural and neurocognitive outcomes.
Methods
Using diffusion tensor imaging, we examined white matter structure in 32 patients with pediatric low-grade glioma (PLGG) (19 with subtentorial location and 13 with supratentorial location) and 32 healthy participants. Indices of intellectual functioning were also evaluated. Radiation was not used to treat this cohort, aged 8–19 years.
Results
We detected evidence of deficits in IQ and compromised supra- and subtentorial white matter in patients relative to healthy children (P < .05). Compromise of supratentorial white matter mediated the impact of treatment for PLGG on IQ. Greater white matter compromise was observed in patients who presented without multiple symptoms, were treated with biopsy/no surgery, had positive neurofibromatosis 1 status, were younger age at diagnosis, and whose parents had lower levels of education (P < .05).
Conclusions
Our findings provide evidence of increased risk of intellectual and white matter compromise in patients treated for PLGG without radiation. We identify a neural origin of cognitive deficit useful for predicting outcome and mitigating long-term adverse effects in pediatric brain tumor patients treated without cranial radiation.
Keywords: IQ, low-grade glioma, supratentorial tumors, subtentorial tumors, white matter
The relationship between white matter and cognitive outcome in pediatric brain tumor patients treated with cranial radiation is well documented.1–5 The reduction in intellectual function that occurs as a late effect of treatment with cranial radiation in childhood6–10 has been associated with changes to white matter structure.4,11,12 In contrast, there is a paucity of literature examining white matter structure in pediatric brain tumor patients not treated with cranial radiation. Indeed, the neurobiological predictors of adverse cognitive function in this group are understudied compared with those in patients requiring more intensive CNS-directed therapy.13 Considering that most patients treated without cranial radiation survive long after their diagnosis, it is critical to identify factors that may influence neurocognitive outcome. In particular, identifying the neural origin of cognitive deficit is essential for predicting outcome and mitigating long-term adverse effects in the group.
Low-grade glioma accounts for 40%–45% of all childhood CNS neoplasms, with the vast majority of patients surviving their disease.14 Treatment for pediatric low-grade glioma (PLGG) is dependent on tumor location. Subtentorial PLGG (ie, cerebellar astrocytoma) is typically treated with maximal surgical resection alone.15,16 Supratentorial PLGG (ie, optic pathway, hypothalamic, thalamic, or third ventricle tumor) is often treated with subtotal resection/biopsy and adjuvant chemotherapy; in some cases multiple surgeries are required.17,18 Cranial radiation is used infrequently to treat PLGG, making this patient population ideal to examine late effects of white matter change in children treated for brain tumor without cranial radiation.
Long-term outcome of patients treated for PLGG can be quite variable, ranging from minimal disability in some children to severe neuropsychological deficits and health issues in others.13 For many with PLGG, these complications of survival can lead to poor academic performance, reduced vocational attainment, and compromised quality of life.13,15,19–30 IQ scores below normative means have been observed within 2 years following PLGG diagnosis.15,20,31 Patients with subtentorial tumors do relatively well on global intellectual measures19,20 but display specific deficits in attention, memory, processing speed, and visual-spatial function.19,23 Supratentorial tumors are associated with greater cognitive disability, as well as hearing impairment, endocrine dysfunction, and seizure disorder.22,24,31 Younger age at diagnosis, presence of neurofibromatosis 1 (NF1), shunt placement, and chemotherapy predict lower intelligence scores in PLGG.24,25 Treatment with cranial radiation is associated with worse outcome.25 As yet, there is limited understanding of the neurobiological underpinning of these deficits.
The scarcity of literature examining white matter structure in patients not treated with cranial radiation has precluded identification of the neurobiological predictors of adverse cognitive function in this group. In the single study we are aware of that examined white matter structure in pediatric brain tumor patients not treated with cranial radiation, patients with subtentorial astrocytoma demonstrated compromised supra- and subtentorial white matter structure compared with healthy children.32 In the present study, we examined intellectual ability and white matter structure in PLGG patients treated without cranial radiation and in healthy children.
We used diffusion tensor imaging (DTI) to measure white matter structure. DTI allows for the visualization of different aspects of tissue microstructure based on water molecule displacement and directionality,33 particularly white matter structure. DTI yields voxelwise maps of several measures, including diffusivity of water movement, tissue anisotropy, and fiber directionality. These maps are based on 3 separate eigenvectors, λ1, λ2, and λ3, calculated by matrix diagonalization.33,34 The eigenvectors are combined to provide the quantitative DTI indices of fractional anisotropy (FA) and mean diffusivity (MD), as well as axial and radial diffusivity (AD and RD, respectively). FA reflects the principal diffusion direction within each voxel and is a ratio of λ1 divided by the sum of λ2 and λ3. FA is considered to reflect myelin structure, as myelin acts as a barrier to free water molecule movement.35 MD measures the magnitude of water diffusion and is the average of λ1, λ2, and λ3.33 MD is thought to reflect both axon and myelin structure.34 The first eigenvector (λ1) measures AD, reflecting diffusion parallel to the axonal fibers. The second and third eigenvectors (λ2 and λ3) represent diffusion perpendicular to axonal fibers.33 Radial diffusivity is the average of λ2 and λ3 and is an overall measure of this perpendicular diffusion.33 Measures of AD and RD are thought to reflect axon and myelin structure, respectively.34 Our first goal was to examine whether white matter compromise mediates poor intellectual outcome in patients with PLGG. We predicted that (i) lower IQ scores and compromised white matter structure would be observed in patients compared with healthy children and (ii) such structural compromise would mediate group effects in intellectual outcome (Fig. 1). Our second goal was to examine specific predictors of outcome in patients treated for PLGG. Hence, we examined the impact of tumor-related, treatment-related, host-related, and environmental variables on white matter.
Fig. 1.
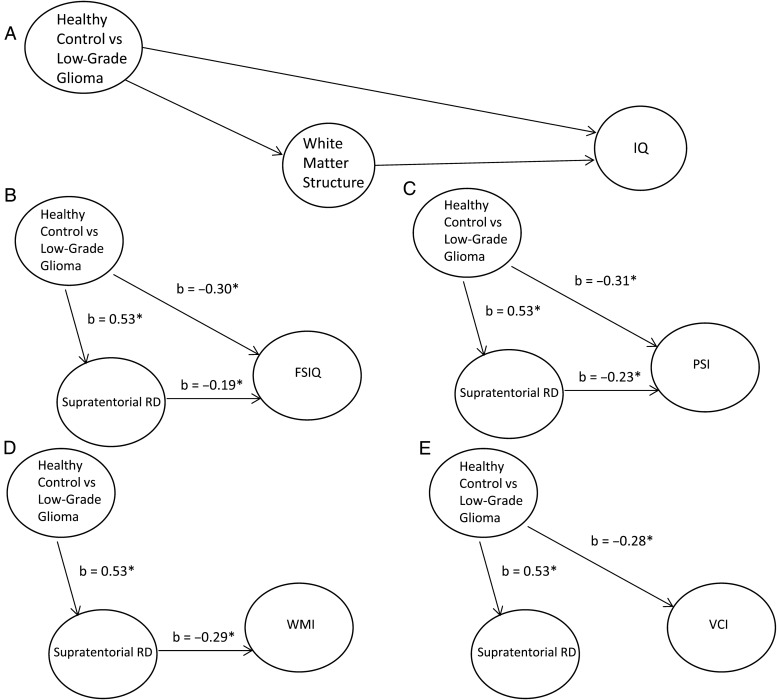
Path analysis. Weights from multiple regression analyses were employed to estimate the strength of relations between variables for the hypothesized model. Treatment for PLGG was assumed to be causally independent of the other variables. We tested whether treatment influenced intelligence directly and/or indirectly via the effect on white matter organization. For each proposed relation, a coefficient was estimated that expressed the changes in standard deviation units of one variable that were associated with a change of one standard deviation in the second variable. Coefficients significantly greater than zero provided evidence for the hypothesized relation between the variables.45 The hypothesized (A) path model and final path models for (B) FSIQ, (C) PSI, (D) WMI, and (E) VCI are shown. *P < .05.
Materials and Methods
Participants
Thirty-two patients with PLGG (19 subtentorial and 13 supratentorial tumors) and 32 healthy children participated in this study. All participants were seen at either The Hospital for Sick Children in Toronto (SickKids), British Columbia Children's Hospital in Vancouver (BCCH), Alberta Children's Hospital in Calgary (ACH), or Children's Hospital of Eastern Ontario in Ottawa (CHEO). Controls were recruited through parent networks and friends and family of the investigators. Participants were excluded from the study if they had a premorbid history of traumatic brain injury, neurological disorder, cerebral palsy, developmental delay, or learning disability. This study conformed to the Tri-Council Ethics Principles and was approved by all institutional research ethics boards.
Participant Characteristics
Participant characteristics are detailed in Table 1. Patients were older than healthy children at time of assessment, F(1, 63) = 6.24, P = .02. Parental (paternal and maternal) education was lower for patients compared with healthy children, F(1, 61) < 9.82, P < .01. There were no differences in sex, χ2(1) = 0.25, P = .40, or handedness, χ2(1) = 1.10, P = .30.
Table 1.
Participant characteristics
| Healthy Control | Low-Grade Glioma | |
|---|---|---|
| n = 32 | n = 32 | |
| Age at assessment, y* | ||
| Mean | 12.31 | 13.99 |
| SD | 2.33 | 3.03 |
| Range | 8.83–17.17 | 8.42–19.12 |
| Mother's education, y* | ||
| Mean | 18.60 | 14.34 |
| SD | 4.61 | 2.60 |
| Range | 12.0–30.0 | 7.0–19.0 |
| Father's education, y* | ||
| Mean | 17.47 | 14.53 |
| SD | 3.77 | 3.61 |
| Range | 10.0–25.0 | 6.0–25.0 |
| Sex, M | 18 (56%) | 16 (50%) |
| Handedness, % right | 87.5 | 84.38 |
*P < .05.
Medical Variables and Assessment Details of Patients
Medical variables and assessment details of patients are described in Table 2 as a function of tumor location. Subtentorial tumor locations were fourth ventricle (n = 2), brainstem (n = 2), cerebral peduncle (n = 1), cerebellar hemispheric (left = 2, right = 2), vermis (n = 6), and leptomeningeal space and infiltrating cerebellum (n = 1). Supratentorial tumor locations were optic pathway (n = 7), midline (hypothalamic = 1, midbrain = 1, thalamus = 1), and parietal lobe (n = 1). Specific location was missing for 6 patients. A number of differences were evident in our sample of patients with sub- versus supratentorial tumors. Patients with subtentorial tumors were younger than patients with supratentorial tumors at time of assessment, F(1, 30) = 9.25, P = .005, but were older at age of diagnosis, F(1, 30) = 11.03, P = .002. Time elapsed from diagnosis to assessment was less for patients treated for subtentorial tumors, F(1, 30) = 25.81, P < .001. More patients treated for subtentorial tumors had multiple presenting symptoms (ie, headaches, vomiting, motor and sensory changes) than patients treated for supratentorial tumors, χ2(1) = 7.42, P = .01. Consistent with current management of PLGG, a greater number of patients with subtentorial tumors were treated with gross total resection compared with patients with supratentorial tumors, χ2(1) = 13.76, P = .003. Further, a greater frequency of patients with subtentorial tumors underwent surgery, χ2(1) = 11.84, P = .003, and had multiple residual complications, χ2(1) = 4.41, P = .04. Many experienced ataxia, which is a common issue following posterior fossa surgery. More patients with supratentorial tumors were treated with chemotherapy, including cisplatin and vincristine, χ2(1) = 4.34, P = .05, and had a positive NF1 status compared with those with subtentorial tumors, χ2(1) = 8.66, P = .006. Notably, all patients with NF1 had abnormalities on MRI, including multiple unidentified bright objects on T2 imaging. No differences in requirement for multiple surgeries or refractory hydrocephalus requiring surgical intervention were detected between the 2 patient groups, χ2(1) > 0.20, P > .50.
Table 2.
Medical variables and assessment details of participants with low-grade glioma
| Subtentorial | Supratentorial | |
|---|---|---|
| n = 19 | n = 13 | |
| Age at assessment, y* | ||
| Mean | 12.79 | 15.75 |
| SD | 2.97 | 2.22 |
| Range | 8.42–18.02 | 12.35–19.12 |
| Age at diagnosis, y* | ||
| Mean | 7.22 | 3.86 |
| SD | 3.12 | 2.26 |
| Range | 1.60–12.33 | 1.05–8.41 |
| Time from diagnosis to assessment, y* | ||
| Mean | 5.58 | 11.89 |
| SD | 3.95 | 2.52 |
| Range | 0.59–13.26 | 8.82–16.76 |
| Duration of symptoms, moa | 15.76 | 9.14 |
| Tumor size, mm2b | 2144.33 | 2400.00 |
| Multiple presenting symptoms* | 15 (78.9%) | 4 (21.1%) |
| Surgical outcome/extent of resection* | ||
| Resection >95% | 13 (68.42%) | 2 (15.38%) |
| 50%–95% of tumor resected | 4 (21.05%) | 4 (30.77%) |
| Biopsy | 2 (10.52%) | 1 (7.69%) |
| No surgery | 0 (0.0) | 7 (46.15%) |
| Number of surgeries | ||
| One or less | 17 (89.47%) | 11 (84.61%) |
| Multiple | 2 (11.76%) | 2 (15.38%) |
| Presence of residual symptoms/complications | 10 (52.63%) | 3 (23.07%) |
| Multiple residual symptoms/complications* | 6 (31.58%) | 0 (0%) |
| Hydrocephalus | ||
| Presence of refractory hydrocephalus | 3 (15.79%) | 3 (23.04%) |
| Chemotherapy* | 3 (15.79%) | 7 (53.85%) |
| NF1 status* | 0 (0.0%) | 5 (38.46%) |
aData were unavailable for 2 subtentorial patients and 6 supratentorial patients.
bTumor size was calculated by multiplying the 2 largest measurements of the tumor from anatomical MRI scan. Measurements are in mm2. Tumor size dimensions were not available for 7 subtentorial patients and 12 supratentorial patients. *P < .05.
Intellectual Measures
The Wechsler Intelligence Scale for Children–Fourth Edition was used to assess intelligence. (The Wechsler Adult Intelligence Scale—Fourth Edition was employed for individuals older than 16 years). The Full Scale Intelligence Quotient (FSIQ) measures overall cognitive functioning, the Verbal Comprehension Index (VCI) measures verbal reasoning and conceptualization abilities, and the Perceptual Reasoning Index (PRI) evaluates the ability to interpret and organize visually presented nonverbal information.36 The Working Memory Index (WMI) measures attention/working memory abilities, and the Processing Speed Index (PSI) evaluates the speed of grapho-motor and mental processing.36
Imaging Data Acquisition
All patients underwent MRI using a protocol previously described.3 Images were acquired on a GE LX 1.5T scanner with 8-channel head coil at SickKids and CHEO, and on a Siemens 1.5T scanner with 8-channel head coil at BCCH and ACH. Diffusion tensor imaging (31 directions for GE, and 30 directions for Siemens) was collected with an echo planar readout: b-value = 1000 s/mm2, echo time (TE) = 84.6 ms, repetition time (TR) = 15 000 ms, field of view (FOV) = 240 mm, matrix = 128 × 128, number of slices = 45–50, slice thickness = 3 mm. A high-resolution T1 image with voxel size 0.937 × 0.937 × 1.5 mm3 was acquired for anatomical reference. The scanning protocol also included a 3D-T1 fast spoiled gradient echo, inversion recovery–prepared sequence: TE/TR = 4.2/10 ms, matrix = 256 × 192, flip angle = 20 degrees, FOV = 240 mm, number of slices = 124. Because we have previously documented signal-to-noise differences across the scanners used in this study,3 type of scanner (GE at SickKids/CHEO vs Siemens at BCCH/ACH) was included as a covariate in analyses of imaging measures.
White Matter Analysis
White matter was segmented (Functional Magnetic Resonance Imaging of the Brain [FMRIB] Software Library–FMRIB Automated Segmentation Tool [FSL-FAST])37 and compartments were used as regions of interest on the T1 scan. White matter was subdivided into 8 supratentorial compartments (bilateral frontal, parietal, temporal, and occipital hemispheric) and 4 subtentorial compartments (pontine, vermal, and bilateral cerebellar hemispheric) based on an anatomical template (Fig. 2).38 Individual T1 scans were registered to DTI acquisition space39,40 to delineate the white matter compartments. Mean values for FA, MD, AD, and RD were acquired regionally in white matter compartments. Estimates of total supratentorial white matter structure were calculated by averaging mean DTI values across the 8 cerebral compartments. Likewise, estimates of total subtentorial white matter structure were calculated by averaging mean DTI values across the 4 posterior fossa compartments.
Fig. 2.
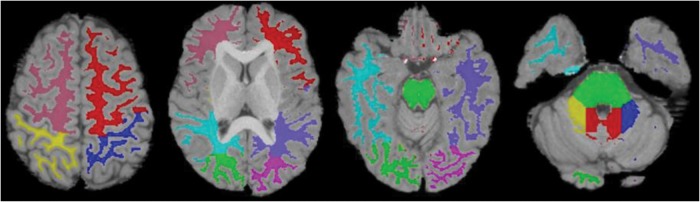
White matter compartments. White matter segmentations (FSL-FAST37) were subdivided into compartments using an anatomically based template modified from Kabani et al38: 8 supratentorial (bilateral frontal, parietal, temporal, and occipital hemispheric) and 4 subtentorial (pontine, vermal, and bilateral cerebellar hemispheric) regions.51 Anatomically divided white matter was registered to DTI space.39,40 In supratentorial white matter, hot pink is left occipital, green is right occipital, purple is left temporal, light blue is right temporal, red is left frontal, pink is right frontal, dark blue is left parietal, and yellow is right parietal. In subtentorial white matter, green is pons, red is vermis, blue is left cerebellum, and yellow is right cerebellum.
Voxelwise Tract-based Spatial Statistics
Tract-based spatial statistics (TBSS) was used for voxelwise analysis.41 FA maps were nonlinearly registered to a standard space image (FMRIB58_FA). A cross-subject mean FA image was created and used to generate a skeleton FA map, thresholded at FA > 0.20, which included only the large fiber tracts. Finally, individual aligned FA maps were projected on the skeleton, and only the maximum FA values along the width of each large fiber tract were considered in subsequent voxelwise analyses. MD, AD, and RD were also evaluated.
Analytic Plan
To identify intellectual differences, we conducted a 2-group (healthy control vs PLGG) multivariate analysis of variance (MANOVA) across IQ scores (FSIQ, VCI, PRI, WMI, PSI), controlling for parental education.
We also conducted 2-group (healthy control vs PLGG) MANOVAs across DTI measures (FA, MD, AD, and RD) to identify structural differences in total supratentorial and total subtentorial white matter, controlling for age at testing and scanner type. Treating supratentorial white matter and subtentorial white matter as single regions of interest reduced potential type 1 error from multiple comparisons.
Voxelwise analyses were then used to identify specific areas of white matter difference between patients and healthy children. TBSS includes a multiple-comparison correction both by creating a “skeleton” FA map, and thus reducing the number of tests, and by controlling for family-wise errors using a permutation methodology. In TBSS, only those voxels common to all subjects were considered in comparing DTI measures across groups, with clusters defined by t > 3. The null distribution of the cluster-size statistic was built up over 5000 random permutations of group membership. Cluster size was thresholded at P < .05, which is fully corrected for multiple comparisons across space.
Next, path models tested whether treatment could influence intelligence directly and/or indirectly via the effect on white matter organization (Fig. 1A). Using a series of regressions, path models were generated for each IQ index with significant group effects. To reduce the number of paths generated and error from multiple comparisons, only the DTI measure with the largest effect size in MANOVAs described above was entered into each regression analysis along with group to predict IQ. Exploratory stepwise regression analyses were then conducted for significant path models to determine the anatomical regions driving relations between white matter and IQ. For each of the 8 hemispheric (or 4 posterior fossa) compartments, the DTI measure with the largest effect size in MANOVAs described above was entered in a stepwise fashion into the regression model predicting IQ, after accounting for parental education and the impact of treatment.
Finally, we used either analysis of variance (ANOVA) for dichotomous variables or regression analyses for continuous variables to test for the impact of specific medical/demographic variables within patients on DTI measures of total supratentorial and total subtentorial white matter. We controlled for scanner type in all analyses. Specifically, we used ANOVAs to test for differences in DTI measures as a function of tumor location (supratentorial vs subtentorial), multiple presenting symptoms (absence/presence), CSF diversion for hydrocephalus (yes/no), treatment with chemotherapy (yes/no), extent of resection (no surgery/biopsy vs subtotal/gross total), requirement for multiple surgeries (yes/no), presence of residual complications (absence/presence), sex (male/female), and NF1 status (positive/negative). Regression analyses were used to test for the contribution of age at diagnosis, tumor size, symptom duration, time since diagnosis, and parental education in predicting DTI measures.
Results
Pediatric Low-Grade Glioma Patients Show Evidence of Poor Intellectual Function and White Matter Compromise
We detected a multivariate group effect across IQ indices (Λ = 0.76, F = 3.33, P = .01). Patients displayed lower standard scores for FSIQ, VCI, PSI, and WMI (P < .05) relative to healthy controls (Table 3).
Table 3.
Group comparisons in IQ, hemispheric white matter, and posterior fossa white matter
| Healthy Controls |
Low-Grade Glioma |
ANOVA |
||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | F | P | |
| IQ | ||||||
| FSIQ | 112.38 | 13.14 | 89.84 | 14.83 | 11.79 | .001 |
| VCI | 112.10 | 15.01 | 93.84 | 15.01 | 5.82 | .02 |
| PRI | 111.45 | 12.06 | 95.45 | 15.16 | 2.85 | .10 |
| PSI | 108.28 | 11.50 | 87.23 | 16.61 | 11.80 | .001 |
| WMI | 103.55 | 9.87 | 89.58 | 15.11 | 4.49 | .04 |
| Supratentorial white matter | ||||||
| FA | 0.40 | 0.02 | 0.38 | 0.03 | 11.45 | .001 |
| MD | 0.000761 | 0.000032 | 0.000809 | 0.000049 | 24.68 | .000006 |
| AD | 0.001102 | 0.000044 | 0.001153 | 0.000048 | 19.04 | .00005 |
| RD | 0.00059 | 0.000029 | 0.000638 | 0.000052 | 24.75 | .000006 |
| Subtentorial white matter | ||||||
| FA | 0.40 | 0.02 | 0.38 | 0.04 | 8.72 | .004 |
| MD | 0.000749 | 0.000044 | 0.000809 | 0.000071 | 17.80 | .00008 |
| AD | 0.001089 | 0.000630 | 0.001151 | 0.000081 | 16.13 | .0002 |
| RD | 0.000579 | 0.000041 | 0.000639 | 0.000072 | 16.64 | .0001 |
Multivariate group effects were also detected across all DTI measures for both total supra- (Λ = 0.70, F = 6.06, P = .0004) and subtentorial white matter (Λ = 0.77, F = 4.30, P = .004). PLGG patients showed decreased FA and increased MD, AD, and RD in supra- and subtentorial white matter structure relative to healthy controls (P < .05; Table 3).
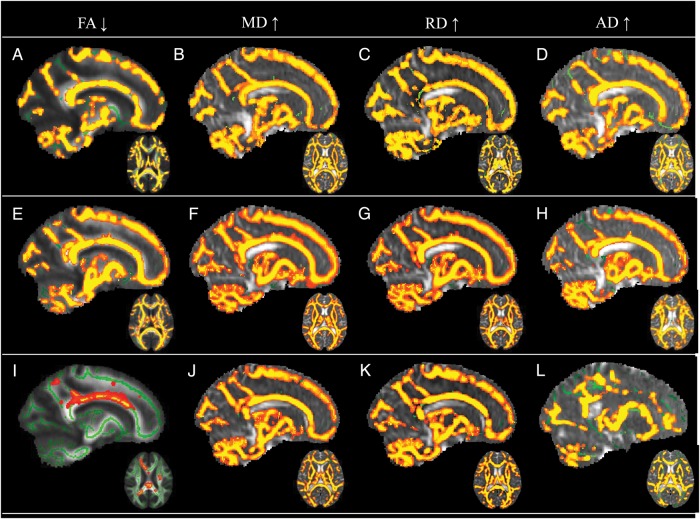
White matter was also compared between PLGG patients and controls using TBSS. Patients and healthy controls were matched for scanner site but not for age at time of assessment; hence this variable was included as a covariate in the analyses. Decreased FA was observed in frontal, parietal, temporal, and occipital lobes bilaterally in PLGG patients relative to healthy control children. FA was also decreased in the genu and splenium of the corpus callosum and the brainstem of patients (Fig. 3, Table 4). The affected cluster size included 61 244 voxels. MD, RD, and AD across these areas were significantly increased in patients.
Fig. 3.
TBSS comparison maps. Skeletons (green) were overlaid on mean FA or merged MD, RD, or AD maps. PLGG tumor patients and controls: clusters (red-yellow) show (A) reduced FA and (B) increased MD, (C) RD, and (D) AD in PLGG tumor patients. Supratentorial tumor patients and controls: clusters show (E) reduced FA and (F) increased MD, (G) RD, and (H) AD in supratentorial tumor patients. Subtentorial tumor patients and controls: clusters show (I) reduced FA and (J) increased MD, (K) RD, and (L) AD in subtentorial tumor patients.
Table 4.
The cluster location and MNI 152 coordinates demonstrating significant differences between patients and controls with respect to FA, MD, RD, and AD
| Cluster Location | MNI 152 Coordinates (X, Y, Z) |
|---|---|
| Left frontal white matter | −13, 54, −7 |
| Right frontal white matter | 35, 31, −5 |
| Left temporal white matter | −53, −27, 5 |
| Right temporal white matter | 42, −7, −34 |
| Left parietal white matter | −25, −53, 4 |
| Right parietal white matter | 20, −44, 4 |
| Left occipital white matter | −16, −73, 1 |
| Right occipital white matter | 21, −91, 1 |
| Corpus callosum genu | 8, 28, −2 |
| Corpus callosum splenium | −13, −40, 14 |
| Brainstem | −8, −31, −12 |
Abbreviation: MNI, Montreal Neurological Institute.
Voxelwise comparisons with healthy controls were also conducted separately for patients with supratentorial tumors and those with subtentorial tumors (Fig. 3). FA was decreased in frontal, parietal, occipital, and temporal lobes bilaterally in patients treated for supratentorial tumors relative to controls. Decreased FA was also apparent in the genu and splenium of the corpus callosum and the brainstem of patients treated for supratentorial tumors. The affected cluster size included 81 825 voxels. Significantly increased MD, RD, and AD were also observed across these areas in patients. Patients with subtentorial tumors showed decreased FA in more circumscribed regions of the left and right superior corona radiata and body of the corpus callosum relative to healthy controls. The affected cluster size included 1549 voxels. In addition to these regions, increased MD, AD, and RD were observed in patients treated for subtentorial tumors across the cerebellum and supratentorial white matter.
Supratentorial White Matter Structure Mediates the Impact of Pediatric Low-Grade Glioma on IQ
As RD showed the largest effect size for group differences (supratentorial RD eta squared (η2) = 0.29; subtentorial RD η2 = 0.21) and is thought to reflect myelin structure,34,35 we used only this index in our subsequent path analyses in order to reduce multiple comparisons. We used beta weights obtained from a series of multiple regression analyses (Table 5) to estimate coefficients describing relations among treatment, RD, and IQ (Fig. 1B–E). First, beta weights from an analysis in which treatment for PLGG was used to predict supratentorial RD served as estimates of the impact of treatment on white matter (Table 5, Model 1). Subsequently, regression analyses were conducted for each IQ index where significant group differences were previously identified (FSIQ, VCI, PSI, WMI) using treatment and supratentorial RD as predictors (Table 5, Models 2–5).
Table 5.
Regression models used in path analyses
| Model R2 | Model F | Model P-value | Standardized Beta Coefficient | Coefficient P-value | |
|---|---|---|---|---|---|
| Model 1, Hemispheric RD | |||||
| Group predicting Hemispheric RDa | 0.27 | 24.75 | .000006 | – | – |
| Group | – | – | – | 0.53 | .000006 |
| Model 2, Full Scale IQ | |||||
| Group and Hemispheric RD predicting FSIQb | 0.14 | 9.68 | .0002 | – | – |
| Group | – | – | – | −0.30 | .007 |
| RD | – | – | – | −0.19 | .05 |
| Model 3, Verbal Comprehension Index | |||||
| 2. Group and Hemispheric RD predicting VCIb | 0.08 | 3.87 | .03 | – | – |
| Group | – | – | −0.28 | .04 | |
| RD | – | – | −0.09 | .45 | |
| Model 4, Processing Speed Index | |||||
| Group and Hemispheric RD predicting PSIb | 0.16 | 8.46 | .001 | – | – |
| Group | – | – | −0.31 | .02 | |
| RD | – | – | −0.23 | .04 | |
| Model 5, Working Memory Index | |||||
| Group and Hemispheric RD predicting WMIb | 0.11 | 5.58 | .006 | – | – |
| Group | – | – | – | −0.13 | .32 |
| RD | – | – | – | −0.29 | .01 |
aAfter accounting for age at testing and scanner type.
bAfter accounting for parental education.
Both treatment for PLGG and supratentorial RD accounted for significant variance in FSIQ (Table 5). Treatment for PLGG also had an indirect effect on FSIQ via its impact on supratentorial RD (Fig. 1B). In further regional evaluation using exploratory stepwise regression, only left frontal RD contributed unique variance to predicting FSIQ (R2 = 0.04, F = 5.24, P = .03, b = −0.20) and was maintained in the model with parental education and group (overall model, R2 = 0.61, F = 30.04, P = .09−10). For PSI, both treatment and RD accounted for significant variance (Table 5). Treatment also had an indirect effect on PSI via its impact on supratentorial RD (Fig. 1C). Only right temporal RD contributed unique variance in predicting PSI (R2 = 0.04, F = 4.84, P = .03, b = −0.25) and was maintained in the model with parental education and group (overall model, R2 = 0.48, F = 17.72, P = .03−7). Notably, only supratentorial RD accounted for significant variance in WMI, with the impact of treatment having an indirect impact through supratentorial RD (Fig. 1D). Only left frontal RD contributed unique variance (R2 = 0.12, F = 13.01, P = .001, b = −0.36) and was maintained in the model with parental education (overall model, R2 = 0.45, F = 23.81, P = .03−7). Supratentorial RD did not have a direct effect on VCI (Fig. 1E). Furthermore, subtentorial RD did not have mediating effects on any of the measures of IQ (P > .05).
The Impact of Specific Medical/Demographic Predictors on Supra- and Subtentorial White Matter in PLGG Patients
In patients only, we examined total supra- and subtentorial white matter RD as a function of specific medical and demographic variables. As noted above, only RD was chosen because the largest effect size was observed for this measure in our prior analyses and in order to reduce multiple comparisons. For both supra- and subtentorial white matter, increased RD was observed in patients who (i) presented without versus with multiple symptoms, (ii) were treated with biopsy/no surgery versus those treated with subtotal/gross total resection, and (iii) had positive NF1 status (F < 3.90, P < .05). Further, younger age at diagnosis and lower parental education predicted increased RD for both supra- and subtentorial white matter compartments (F < 4.40, P < .05). No significant effects on supra- or subtentorial RD were evident as a function of (i) tumor location, (ii) tumor size, (iii) symptom duration, (iv) residual complications, (v) requirement for multiple surgeries, (vi) hydrocephalus requiring CSF diversion, (vii) treatment with chemotherapy, (viii) time since diagnosis, or (ix) gender (F > 3.63, P > .05).
Conclusions
The relationship between white matter and cognitive outcome has, until now, been examined only in pediatric brain tumor patients treated with cranial radiation.1,2,4,11,12 The present study was the first to examine whether estimates of white matter compromise predict intellectual deficit in patients with PLGG treated without radiation. Consistent with previous reports, we detected evidence of compromised supra- and subtentorial white matter32 and deficits in IQ15,20,31 in the patient cohort relative to controls. As a novel finding, we showed that the impact of treatment for PLGG on intellectual outcome is mediated by compromise of supratentorial white matter. Our findings provide evidence of structural and functional compromise in patients treated for PLGG in the absence of radiation.
We found that white matter compromise, as inferred from DTI indices of decreased fiber orientation and increased water diffusion, may mediate adverse intellectual outcome in patients treated for low-grade glioma. In particular, deficits in full-scale intelligence were best predicted by compromise to left frontal white matter, and deficits in information processing speed were best accounted for by injury to right temporal white matter. The biological mechanism relating treatment for PLGG to adverse intellectual outcome may involve microscopic damage to normal-appearing white matter. As frontal networks are most associated with the higher-level reasoning required for global measures of intelligence in children,42 tissue loss from microscopic injury in frontal white matter likely hinders core cognitive functioning that supports intellectual performance. Likewise, microscopic damage to temporal white matter may impact networks required for efficiency in cognitive processing. Indeed, right temporal-parietal white matter predicts the development of information processing speed,43 and in turn, increases in information processing speed during childhood predict intellectual development.44,45 These findings support the theory that white matter structure mediates the impact of treatment for PLGG on IQ and may act as a neurobiological predictor of adverse cognitive function in patients treated for brain tumor without radiation. Notably, the effects of treatment for PLGG on working memory were indirect, mediated solely through left frontal white matter. This outcome is consistent with our previous report that frontal white matter may mediate verbal working memory.3 Taken together, our findings indicate that white matter compromise is sufficient to mediate cognitive deficits, irrespective of treatment with cranial radiation. Furthermore, mediation of cognitive deficits appears to be carried out in a network-specific manner.
Consistent with prior work, we documented white matter compromise in PLGG patients.32 Patients displayed widespread white matter compromise across the cerebral hemispheres, large tracts, and subtentorial regions compared with control participants. That we observed structural compromise in patients who were not treated with cranial radiation is evidence that other factors than this treatment modality can result in white matter damage.32 Considering that for many patients tumor location was in midline supratentorial regions, such widespread hemispheric compromise is not surprising. Hydrocephalus likely plays a role in tissue damage.32 CSF accumulation increases intracranial pressure and produces mechanical stress that decreases cerebral blood flow, reduces the availability of neurotransmitters, damages axons and myelin, and renders neurons dysfunctional.46 Further, shunting procedures cause direct structural damage and increase the risk for postoperative complications.47 Moreover, the potentially neurotoxic impact of treatment with chemotherapy must always be considered.
We observed greater white matter compromise in patients who were treated with biopsy or no surgery. Infiltration into healthy tissue by the tumor or compression and subsequent damage of healthy tissue from mass effect likely contributes to insult.32,48 We also observed that patients with NF1 showed greater evidence of white matter insult. The NF1 mutation is independently associated with white matter damage, and likely contributed to compromise in our patients carrying the mutation.49,50 Further, younger age at diagnosis and lower parental education—variables that we observed to predict white matter compromise—are known risk factors for poor outcome.
Although we found that PLGG patients showed poorer outcome than healthy children, we note some limitations that warrant caution in interpreting our results. First, mean IQ for the control sample was almost one standard deviation above the normative mean. Hence, the differences between the control and patient groups are likely greater than would be expected if a more representative control sample, such as patient siblings, were available. Second, because of our relatively small patient sample, we did not have the power to consider all medical/demographic variables simultaneously in their impact on white matter. Hence we were unable to identify each variable's unique contribution in predicting patient outcome. Furthermore, many of our variables were confounded with each other. For example, we did not observe any effects of tumor location. In our sample, patients with supratentorial tumors were seen for assessment at a longer interval from diagnosis and were younger at diagnosis, and more were treated with chemotherapy compared with those with subtentorial tumors. Such confounds may have limited our power to detect effects. Finally, given the relatively network-specific effects that we observed, in the future it would be important to examine specific cognitive functions, rather than just intelligence measures.
This study provides evidence for white matter compromise mediating intellectual outcome in a sample of pediatric brain tumor patients not treated with radiation. Such evidence is important in challenging the current focus solely on radiation-induced injury in these vulnerable patients. Further, that a neurobiological substrate for late effects in children with PLGG is similar to that seen in those with more aggressive tumors treated with radiation is further support for the notion that children with brain tumors be viewed on a continuum of risk, and patients with PLGG be included in clinics and support programs that follow them over time.13 Finally, as we move into an era focused on genomics and personalized medicine, cognitive rehabilitation, neuroprotection, and brain repair, the evidence that PLGG is associated with white matter compromise that predicts cognitive outcome helps us to see that interventions targeting neurotoxicity in pediatric brain tumor patients must broadly consider the multiple ways in which injury to white matter can occur.
Funding
This research was funded by the Pediatric Brain Tumor Foundation of the United States, the C17 Research Network, and the Canadian Cancer Society.
Conflict of interest statement. The authors have no conflicts of interest to declare.
References
- 1.Brinkman TM, Reddick WE, Luxton J, et al. Cerebral white matter integrity and executive function in adult survivors of childhood medulloblastoma. Neuro Oncol. 2012;14(Suppl 4):iv25–36. doi: 10.1093/neuonc/nos214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmer SL, Glass JO, Li Y, et al. White matter integrity is associated with cognitive processing in patients treated for a posterior fossa brain tumor. Neuro Oncol. 2012;14(9):1185–1193. doi: 10.1093/neuonc/nos154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Law N, Bouffet E, Laughlin S, et al. Cerebello-thalamo-cerebral connections in pediatric brain tumor patients: impact on working memory. Neuroimage. 2011;56(4):2238–2248. doi: 10.1016/j.neuroimage.2011.03.065. [DOI] [PubMed] [Google Scholar]
- 4.Mabbott DJ, Noseworthy MD, Bouffet E, et al. Diffusion tensor imaging of white matter after cranial radiation in children for medulloblastoma: correlation with IQ. Neuro Oncol. 2006;8(3):244–252. doi: 10.1215/15228517-2006-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulhern RK, White HA, Glass JO, et al. Attentional functioning and white matter integrity among survivors of malignant brain tumors of childhood. J Int Neuropsychol Soc. 2004;10(2):180–189. doi: 10.1017/S135561770410204X. [DOI] [PubMed] [Google Scholar]
- 6.Spiegler BJ, Bouffet E, Greenberg ML, et al. Change in neurocognitive functioning after treatment with cranial radiation in childhood. J Clin Oncol. 2004;22(4):706–713. doi: 10.1200/JCO.2004.05.186. [DOI] [PubMed] [Google Scholar]
- 7.Copeland DR, deMoor C, Moore BD, 3rd, et al. Neurocognitive development of children after a cerebellar tumor in infancy: a longitudinal study. J Clin Oncol. 1999;17(11):3476–3486. doi: 10.1200/JCO.1999.17.11.3476. [DOI] [PubMed] [Google Scholar]
- 8.Ris MD, Packer R, Goldwein J, et al. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a Children's Cancer Group study. J Clin Oncol. 2001;19(15):3470–3476. doi: 10.1200/JCO.2001.19.15.3470. [DOI] [PubMed] [Google Scholar]
- 9.Palmer SL, Goloubeva O, Reddick WE, et al. Patterns of intellectual development among survivors of pediatric medulloblastoma: a longitudinal analysis. J Clin Oncol. 2001;19(8):2302–2308. doi: 10.1200/JCO.2001.19.8.2302. [DOI] [PubMed] [Google Scholar]
- 10.Palmer SL, Gajjar A, Reddick WE, et al. Predicting intellectual outcome among children treated with 35–40 Gy craniospinal irradiation for medulloblastoma. Neuropsychology. 2003;17(4):548–555. doi: 10.1037/0894-4105.17.4.548. [DOI] [PubMed] [Google Scholar]
- 11.Reddick WE, White HA, Glass JO, et al. Developmental model relating white matter volume to neurocognitive deficits in pediatric brain tumor survivors. Cancer. 2003;97(10):2512–2519. doi: 10.1002/cncr.11355. [DOI] [PubMed] [Google Scholar]
- 12.Khong PL, Leung LH, Fung AS, et al. White matter anisotropy in post-treatment childhood cancer survivors: preliminary evidence of association with neurocognitive function. J Clin Oncol. 2006;24(6):884–890. doi: 10.1200/JCO.2005.02.4505. [DOI] [PubMed] [Google Scholar]
- 13.Ris MD, Beebe DW. Neurodevelopmental outcomes of children with low-grade gliomas. Dev Disabil Res Rev. 2008;14(3):196–202. doi: 10.1002/ddrr.27. [DOI] [PubMed] [Google Scholar]
- 14.Strother DR, Pollack IF, Fisher PG, et al. Tumors of the Central Nervous System. 4th ed. Philidelphia, PA: Lippincott, Williams, & Wilkins; 2002. [Google Scholar]
- 15.Turner CD, Chordas CA, Liptak CC, et al. Medical, psychological, cognitive and educational late-effects in pediatric low-grade glioma survivors treated with surgery only. Pediatr Blood Cancer. 2009;53(3):417–423. doi: 10.1002/pbc.22081. [DOI] [PubMed] [Google Scholar]
- 16.Rashidi M, DaSilva VR, Minagar A, et al. Nonmalignant pediatric brain tumors. Curr Neurol Neurosci Rep. 2003;3(3):200–205. doi: 10.1007/s11910-003-0079-9. [DOI] [PubMed] [Google Scholar]
- 17.Walker DA, Liu J, Kieran M, et al. A multi-disciplinary consensus statement concerning surgical approaches to low-grade, high-grade astrocytomas and diffuse intrinsic pontine gliomas in childhood (CPN Paris 2011) using the Delphi method. Neuro Oncol. 2013;15(4):462–468. doi: 10.1093/neuonc/nos330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jakacki RI, Bouffet E, Adamson PC, et al. A phase 1 study of vinblastine in combination with carboplatin for children with low-grade gliomas: a Children's Oncology Group phase 1 consortium study. Neuro Oncol. 2011;13(8):910–915. doi: 10.1093/neuonc/nor090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinlin M, Imfeld S, Zulauf P, et al. Neuropsychological long-term sequelae after posterior fossa tumour resection during childhood. Brain. 2003;126(Pt 9):1998–2008. doi: 10.1093/brain/awg195. [DOI] [PubMed] [Google Scholar]
- 20.Beebe DW, Ris MD, Armstrong FD, et al. Cognitive and adaptive outcome in low-grade pediatric cerebellar astrocytomas: evidence of diminished cognitive and adaptive functioning in National Collaborative Research Studies (CCG 9891/POG 9130) J Clin Oncol. 2005;23(22):5198–5204. doi: 10.1200/JCO.2005.06.117. [DOI] [PubMed] [Google Scholar]
- 21.Brown TM, Ris MD, Beebe D, et al. Factors of biological risk and reserve associated with executive behaviors in children and adolescents with spina bifida myelomeningocele. Child Neuropsychol. 2008;14(2):118–134. doi: 10.1080/09297040601147605. [DOI] [PubMed] [Google Scholar]
- 22.Aarsen FK, Van Dongen HR, Paquier PF, et al. Long-term sequelae in children after cerebellar astrocytoma surgery. Neurology. 2004;62(8):1311–1316. doi: 10.1212/01.wnl.0000120549.77188.36. [DOI] [PubMed] [Google Scholar]
- 23.Levisohn L, Cronin-Golomb A, Schmahmann JD. Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain. 2000;123(Pt 5):1041–1050. doi: 10.1093/brain/123.5.1041. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong GT, Conklin HM, Huang S, et al. Survival and long-term health and cognitive outcomes after low-grade glioma. Neuro Oncol. 2011;13(2):223–234. doi: 10.1093/neuonc/noq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein M, Heimans JJ, Aaronson NK, et al. Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study. Lancet. 2002;360(9343):1361–1368. doi: 10.1016/s0140-6736(02)11398-5. [DOI] [PubMed] [Google Scholar]
- 26.Aaronson NK, Taphoorn MJ, Heimans JJ, et al. Compromised health-related quality of life in patients with low-grade glioma. J Clin Oncol. 2011;29(33):4430–4435. doi: 10.1200/JCO.2011.35.5750. [DOI] [PubMed] [Google Scholar]
- 27.Benesch M, Lackner H, Sovinz P, et al. Late sequela after treatment of childhood low-grade gliomas: a retrospective analysis of 69 long-term survivors treated between 1983 and 2003. J Neurooncol. 2006;78(2):199–205. doi: 10.1007/s11060-005-9091-z. [DOI] [PubMed] [Google Scholar]
- 28.Due-Tonnessen BJ, Helseth E, Scheie D, et al. Long-term outcome after resection of benign cerebellar astrocytomas in children and young adults (0-19 years): report of 110 consecutive cases. Pediatr Neurosurg. 2002;37(2):71–80. doi: 10.1159/000065108. [DOI] [PubMed] [Google Scholar]
- 29.Fouladi M, Wallace D, Langston JW, et al. Survival and functional outcome of children with hypothalamic/chiasmatic tumors. Cancer. 2003;97(4):1084–1092. doi: 10.1002/cncr.11119. [DOI] [PubMed] [Google Scholar]
- 30.Tow SL, Chandela S, Miller NR, et al. Long-term outcome in children with gliomas of the anterior visual pathway. Pediatr Neurol. 2003;28(4):262–270. doi: 10.1016/s0887-8994(02)00628-8. [DOI] [PubMed] [Google Scholar]
- 31.Ris MD, Beebe DW, Armstrong FD, et al. Cognitive and adaptive outcome in extracerebellar low-grade brain tumors in children: a report from the Children's Oncology Group. J Clin Oncol. 2008;26(29):4765–4770. doi: 10.1200/JCO.2008.17.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rueckriegel SM, Driever PH, Blankenburg F, et al. Differences in supratentorial damage of white matter in pediatric survivors of posterior fossa tumors with and without adjuvant treatment as detected by magnetic resonance diffusion tensor imaging. Int J Radiat Oncol Biol Phys. 2010;76(3):859–866. doi: 10.1016/j.ijrobp.2009.02.054. [DOI] [PubMed] [Google Scholar]
- 33.Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR in biomedicine. 1995;8(7–8):333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- 34.Song SK, Sun SW, Ramsbottom MJ, et al. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 35.Song SK, Yoshino J, Le TQ, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26(1):132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 36.Wechsler D. The Psychological Corporation. San Antonio, TX: 2003. Wechsler Intelligence Scale for Children Fourth Edition (WISC-IV) [Google Scholar]
- 37.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE transactions on medical imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 38.Kabani NJ, Sled JG, Chertkow H. Magnetization transfer ratio in mild cognitive impairment and dementia of Alzheimer's type. Neuroimage. 2002;15(3):604–610. doi: 10.1006/nimg.2001.0992. [DOI] [PubMed] [Google Scholar]
- 39.Woods RP, Grafton ST, Holmes CJ, et al. Automated image registration: I. General methods and intrasubject, intramodality validation. J Comput Assist Tomogr. 1998;22(1):139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- 40.Woods RP, Grafton ST, Watson JD, et al. Automated image registration: II. Intersubject validation of linear and nonlinear models. J Comput Assist Comogr. 1998;22(1):153–165. doi: 10.1097/00004728-199801000-00028. [DOI] [PubMed] [Google Scholar]
- 41.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 42.Shaw P, Greenstein D, Lerch J, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440(7084):676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 43.Mabbott DJ, Noseworthy M, Bouffet E, et al. White matter growth as a mechanism of cognitive development in children. Neuroimage. 2006;33(3):936–946. doi: 10.1016/j.neuroimage.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 44.Luciano M, Wright MJ, Geffen GM, et al. A genetic investigation of the covariation among inspection time, choice reaction time, and IQ subtest scores. Behavior Genetics. 2004;34(1):41–50. doi: 10.1023/B:BEGE.0000009475.35287.9d. [DOI] [PubMed] [Google Scholar]
- 45.Kail R, Park YS. Processing time, articulation time, and memory span. J Exp Child Psychol. 1994;57(2):281–291. doi: 10.1006/jecp.1994.1013. [DOI] [PubMed] [Google Scholar]
- 46.Del Bigio MR. Neuropathological changes caused by hydrocephalus. Acta neuropathologica. 1993;85(6):573–585. doi: 10.1007/BF00334666. [DOI] [PubMed] [Google Scholar]
- 47.Gopalakrishnan CV, Dhakoji A, Menon G, et al. Factors Predicting the Need for Cerebrospinal Fluid Diversion following Posterior Fossa Tumor Surgery in Children. Pediatr Neurosurg. 2012 doi: 10.1159/000343009. [DOI] [PubMed] [Google Scholar]
- 48.Dennis M, Spiegler BJ, Fitz CR, et al. Brain tumors in children and adolescents—II. The neuroanatomy of deficits in working, associative and serial-order memory. Neuropsychologia. 1991;29(9):829–847. doi: 10.1016/0028-3932(91)90050-i. [DOI] [PubMed] [Google Scholar]
- 49.Karlsgodt KH, Rosser T, Lutkenhoff ES, et al. Alterations in white matter microstructure in neurofibromatosis-1. PLoS One. 2012;7(10):e47854. doi: 10.1371/journal.pone.0047854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferraz-Filho JR, da Rocha AJ, Muniz MP, et al. Diffusion tensor MR imaging in neurofibromatosis type 1: expanding the knowledge of microstructural brain abnormalities. Pediatr Radiol. 2012;42(4):449–454. doi: 10.1007/s00247-011-2274-1. [DOI] [PubMed] [Google Scholar]
- 51.Mabbott DJ, Rovet J, Noseworthy MD, et al. The relations between white matter and declarative memory in older children and adolescents. Brain Res. 2009;1294:80–90. doi: 10.1016/j.brainres.2009.07.046. [DOI] [PubMed] [Google Scholar]