Abstract
Background
Glioma follow-up is based on MRI parameters, which are correlated with survival. Although established criteria are used to evaluate tumor response, radiological markers may be confounded by differences in instrumentation including the magnetic field strength. We assessed whether MRIs obtained at 3 Tesla (T) and 1.5T provided similar information.
Methods
We retrospectively compared imaging features of 30 consecutive patients with WHO grades II and III gliomas who underwent MRI at 1.5T and 3T within a month of each other, without any clinical changes during the same period. We compared lesion volumes on fluid attenuation inversion recovery (FLAIR), ratio of cerebral blood volume (rCBV) on perfusion-weighted imaging, contrast-to-noise ratio (CNR) on FLAIR, and on post-gadolinium 3D T1-weighted sequences between 1.5T and 3T using intraclass correlation coefficient (ICC). Concordance between observers within and between modalities was evaluated using weighted-kappa coefficient (wκ).
Results
The mean ± SD delay between modalities (1.5T and 3T MRI) was 8.6 ± 5.6 days. Interobserver/intraobserver concordance for lesion volume was almost perfect for 1.5T (ICC = 0.96/0.97) and 3T (ICC = 0.99/0.98). Agreement between observers for contrast enhancement was excellent at 1.5T (wκ = 0.92) and 3T (wκ = 0.92). The tumor CNR was significantly higher for FLAIR at 1.5T (P < .001), but it was higher at 3T (P = .012) for contrast enhancement. Correlations between modalities for lesion volume (ICC = 0.97) and for rCBV values (ICC = 0.92) were almost perfect.
Conclusions
In the follow-up of WHO grades II and III gliomas, 1.5T and 3T provide similar MRI features, suggesting that monitoring could be performed on either a 1.5 or a 3T MR magnet.
Keywords: glioma, MRI, neuroimaging, observer variation, reproducibility of results
In neuro-oncology, monitoring of cerebral glioma is based mainly on clinical and imaging follow-up. Identification of oncological treatment efficacy depends on an objective response rate, which can be established by tumor changes on MR imaging. The follow-up of infiltrative gliomas in adults (WHO grades II, III, and IV) requires an MRI every 3 to 6 months, depending on tumor grade of malignancy, to track signs of tumor progression and/or transformation toward a higher grade of malignancy. Tumor response assessment is based on MRI criteria,1–3 such as tumor size evaluations4–6 or enhancement characteristics.7 In the absence of clear-cut malignant transformation, such as the emergence of new contrast enhancement, the progression of WHO grade II gliomas is challenging because it is based on small, incremental, and asymptomatic increases in size on serial T2-weighted MRIs.4,8,9
High-field MRI, especially 3 Tesla (T) MR units, is increasingly available10 and routinely performed on patients with brain disorders including gliomas. Studies have shown that 3T MR is more sensitive than 1.5T MR for detecting brain lesions.11–13 If the magnetic field has an impact on the measured tumor size or the detection of contrast enhancement, then any infiltrative glioma, and particularly grades II and III gliomas, should theoretically be monitored at a given magnetic field strength (either 1.5T or 3T). This may avoid erroneously considering a glioma as progressive when switching from 1.5T to 3T or, conversely, missing a progression when switching from 3T to 1.5T. Recommendations to date neither mention the MR field strength that should be used nor indicate how to handle a change in MR field strength during monitoring of WHO grades II and III gliomas. Aside from clinical care, changes due to MR field strengths may also induce variability in measurements of tumor progression that would bias therapeutic trials using MR-imaging endpoints.14
To shed some light on these issues, we determined if there were any differences between 3T and 1.5T MR scans for the main imaging features of WHO grades II and III gliomas. We further compared the quantitative parameters extracted from first-pass gadolinium-perfusion MR between these 2 magnetic field strengths to determine whether patients harboring a glioma could be monitored regardless of whether a 1.5 or 3T MR unit is used.
Materials and Methods
The Institutional Review Board approved this observational study, and informed consent was obtained from all participants.
Selection Criteria
Over a 6-month period, we retrospectively identified (from a prospectively maintained database) all consecutive adult patients with a diagnosis of supratentorial WHO grade II or III glioma based on pathological examination according to the WHO classification of the CNS tumors.15 We were searching for patients who had undergone both 1.5 and 3T MRI less than one month apart, with index MRI qualifying for the study and no clinical changes between the 2 exams regardless of previous oncological treatments or pathological or molecular subtype. Scanning participants at 3T MRI (new 3T MR unit installed at the start of the study period beside the 1.5T MR unit) was performed in order to obtain a baseline MR exam for further follow-up. The 3T MR unit was used thereafter for all participants who were previously followed on the 1.5T MR unit.
Image Acquisition
MR images were acquired with a 1.5T (SignaEchoSpeed, GE Healthcare) and a 3T (MR750 Discovery, GE Healthcare) scanner using a 16-channel phased-array head coil. Both 1.5T and 3T exams were scheduled within a month of each other, and the sequence of the 2 exams depended on availability of each MR unit. For both modalities, imaging included fluid attenuation inversion recovery (FLAIR), axial T1-weighted (-w) sequences, perfusion-weighted imaging (PWI), and postcontrast 3D T1-w sequences (Fig. 1). PWI was obtained using a T2*-weighted echo-planar sequence after a bolus (5–7 mL/s of 0.1 mmol/kg of body weight) of gadoteric acid (Guerbet. Postcontrast 3D T1-w was obtained approximately 3 minutes after contrast injection. Total acquisition time was 13 minutes for a 1.5T examination and 14 minutes for a 3T examination.
Fig. 1.
Patients with glioma at 1.5T (left column) and 3T (right column). A and B. Axial FLAIR sequence at 1.5T (A) and 3T (B) of a 51-year-old woman with a left frontal glioma (WHO grade II) treated with radiation therapy 9 years before. Time interval between examinations: 3 days. C, D, E and F. Post-contrast 3D T1-w (C, D), and perfusion images (E, F) of a 56-year-old patient with a progressing grade II oligoastrocytoma treated 10 years before with surgery and radiation therapy, showing nodular contrast enhancement (white arrows) at 1.5 (C) and 3-T (D). Focal rCBV increase visible as a “hot spot” (high values in red, low values in blue) on co-registered perfusion parametric map and post-contrast 3D T1-w images (black arrows) at 1.5 (E) and 3T (F). Time interval between examinations: 7 days.
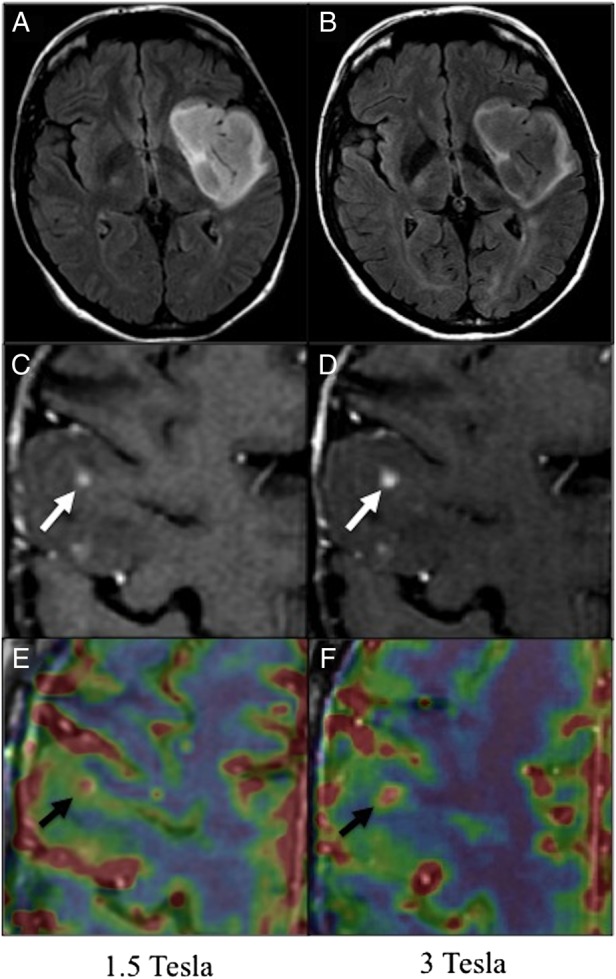
Image Analysis
Two radiologists (R.S.S., 10 years of experience with 5 years specialized in neuro-oncology; L.T., 5 years of experience with no specialization in neuro-oncology; they had been working together for <6 months at the time of the study), who were blinded to participants' clinical files, independently analyzed 1.5T and 3T MRIs separately (the order of analysis was random) on a dedicated workstation (Advantage Windows, GE Healthcare) displaying FLAIR, T1-w, and postcontrast 3D T1-w images of 1.5 or 3T examinations at the same time. Three months later, one radiologist (R.S.S.) reanalyzed the same panel of images in random order to assess intraobserver variability. Interactive tools based on FLAIR signal intensity were used to obtain lesion volume (mL). Semiautomated 3D segmentation included signal intensity thresholding within a 3D mask encompassing the area of hyperintensity, morphometric filtering, and manual contouring. Contrast enhancement was analyzed using a simplified scale (no enhancement, faint and patchy contrast enhancement, nodular or ring-like contrast enhancement).7 In the case of discrepancies between readers, the final score was reached by consensus. The lesion's contrast-to-noise ratio16 on FLAIR sequences (CNRFLAIR) was measured. For this purpose, 1.5T and 3T FLAIR images were coregistered using automated 3D rigid registration software (Integrated Registration, GE Healthcare), checked visually, and corrected manually when necessary. Then, 2D circular (10 mm diameter) regions of interest (ROIs) were positioned in the lesion, in the nontumoral contralateral white matter, and in the background with consensus by the 2 observers. This set of 3 ROIs was projected on all images (1.5T and 3T) to insure similar ROI placement. Except for ROI size, which was adapted to the size of the enhancing portion of the lesion, a similar method was used to measure lesion enhancement after contrast administration (CNRCONTRAST). PWI data were postprocessed using Brain-Stat Arterial Input Function software (READY View, GE Healthcare) for an automated generation of cerebral blood volume (CBV) maps. These were obtained by circular deconvolution of the tissue concentration time course using an arterial input function from contralateral arteries. On CBV maps obtained with 1.5T and 3T images, a ROI was centered on the hot spots (peak CBV value) within the lesion and then copied to the contralateral nontumoral tissue at 1.5 and 3T in order to compute CBV ratios (rCBV).
Finally, the effect of MR field strength on participants' response to oncological treatments was analyzed. Both radiologists and a neurosurgeon (J.P., 7 years of experience) jointly compared the index MRIs reports and clinical features to participants' previous (3 to 6 months prior to index MRIs) and next (3 to 6 months after index MRIs) evaluations according to Response Assessment in Neuro-Oncology (RANO) criteria for diffuse low-grade1 and high-grade gliomas.2
Statistical Analysis
The characteristics of participants and lesions are presented as numbers and percentages for qualitative variables and means ± SDs for quantitative variables. Interobserver and intraobserver variability was assessed using Cohen's quadratic weighted kappa coefficient (wκ) for qualitative ordinal ranked data.17 Intraclass coefficient (ICC) was used for quantitative variables.18 Agreement was considered as moderate for wκ = 0.41–0.6, substantial for wκ = 0.61–0.8, and almost perfect for wκ = 0.81–1 or ICC > 0.8.19 Observer 1's (most experienced) results were considered as the reference for 1.5 versus 3T comparisons of volumes and contrast enhancement. A Wilcoxon matched-pairs signed-rank test was performed to compare variables between 1.5T and 3T modalities. A 2-sided P value < .05 was considered statistically significant. Statistical analysis was performed with the SPSS statistical software package, version 22 (IBM, SPSS Statistics).
Results
Participant and Tumor Characteristics
Thirty participants fulfilled the inclusion criteria and comprised the study group. Their characteristics are summarized in Table 1. Twenty-six (87%) participants harbored a WHO grade II glioma, and 4 (13%) harbored a WHO grade III glioma. Most partilcipants (n = 22, 73%) had the1.5T MRI before 3T MRI. Mean interval (±SD) between 1.5 and 3T MR examinations was 8.6 (±5.6) days. None of the participants experienced clinical worsening or epileptic seizures in the interval between the 2 MRIs.
Table 1.
Participant and lesion characteristics
| n = 30 | |
|---|---|
| Age, years, mean ± SD (Range) | 48 ± 13 |
| Sex | |
| Male | 19 (63) |
| Female | 11 (37) |
| Tumor histopathological subtype | |
| Astrocytoma | 8 (27) |
| Glioma with oligodendroglial component | 22 (73) |
| Oncological treatments | |
| None | 4 (13) |
| Surgery alone | 2 (7) |
| Radiation therapy alone | 6 (20) |
| Surgery + radiation therapy | 8 (27) |
| Surgery + chemotherapy | 1 (3) |
| Radiation therapy + chemotherapy | 2 (7) |
| Surgery + radiation therapy + chemotherapy | 7 (23) |
| Number of oncological treatments (When treated) | |
| 1 | 8 (27) |
| 2 | 11 (37) |
| 3 | 7 (23) |
Abbreviation: SD, standard deviation.
Results are expressed as number of participants (percentages), unless specified.
Interobserver and Intraobserver Variability
Interobserver and intraobserver agreements were excellent for lesion volumes at either 1.5T (ICCinter = 0.96; 95% CI, 0.92–0.98/ICCintra = 0.97; 95% CI, 0.95–0.99) or 3T (ICCinter = 0.99; 95% CI, 0.98–1/ICCintra = 0.98; 95% CI, 0.95–0.99) (Table 2). Regarding the classification of focal areas of contrast enhancement, intraobserver reproducibility was perfect (wκ = 1), and interobserver reproducibility was almost perfect (wκ = 0.92; 95% CI, 0.73–1) for both 1.5T and 3T (discrepancies observed in 2 participants at 1.5T and 2 at 3T) MRIs.
Table 2.
Morphological imaging analysis at 1.5 and 3Tesla in the 30 participants studied
| Reader 1 First Session |
Reader 1 Second Session |
Reader 2 |
||||
|---|---|---|---|---|---|---|
| 1.5T | 3T | 1.5T | 3T | 1.5T | 3T | |
| Lesion volume on FLAIR, mL ± SD | 44.3 ± 33.1 | 44.7 ± 37.3 | 42.8 ± 27.4 | 43.4 ± 30.6 | 40.7 ± 25.2 | 42.3 ± 34.3 |
| Contrast enhancement n (%) | ||||||
| No enhancement | 18 (60) | 18 (60) | 18 (60) | 18 (60) | 19 (63) | 19 (63) |
| Faint and patchy | 6 (20) | 6 (20) | 6 (20) | 6 (20) | 6 (20) | 6 (20) |
| Nodular or ring like | 6 (20) | 6 (20) | 6 (20) | 6 (20) | 5 (17) | 5 (17) |
Abbreviations: FLAIR, fluid-attenuated inversion recovery; SD, standard deviation; T, Tesla.
Results are expressed as number of participants (percentages), unless specified.
Comparison Between 1.5 and 3 Tesla
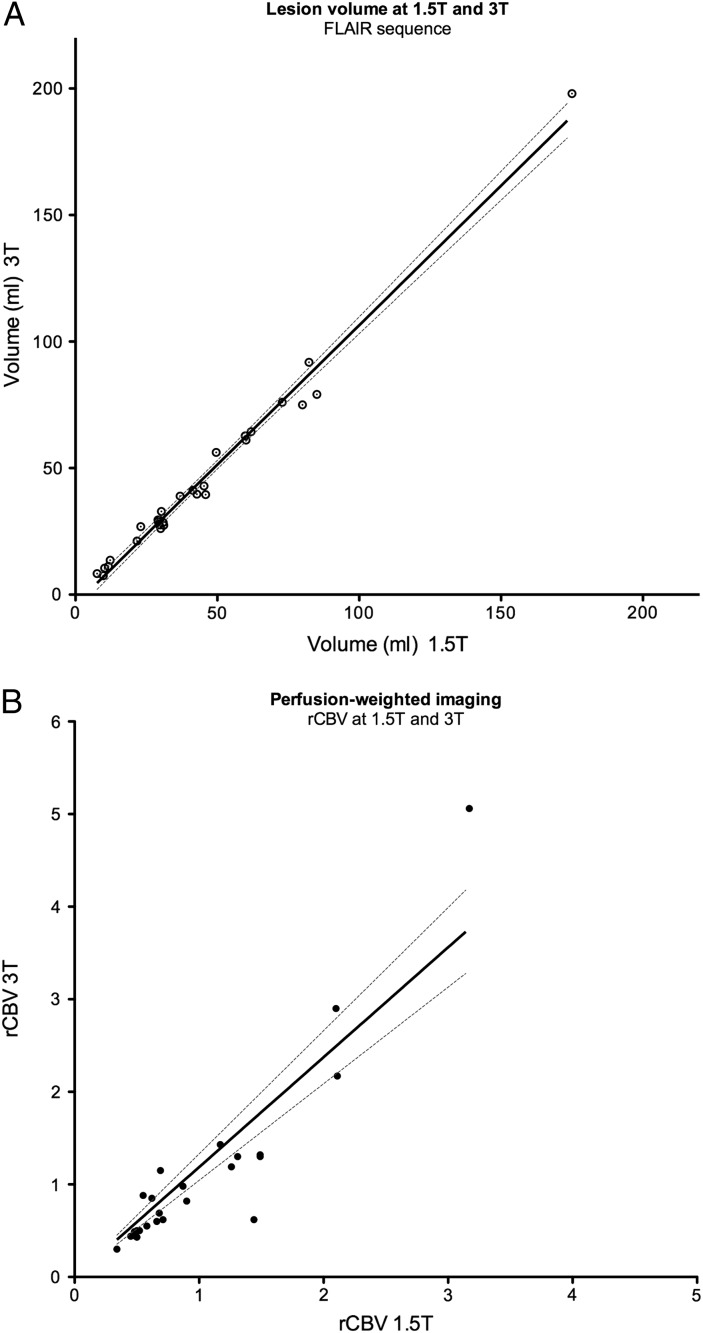
Tumor volumes obtained at 1.5T and 3T were almost perfectly correlated (ICC = 0.97; 95% CI, 0.95–0.99) (Fig. 2A), despite the fact that CNRFLAIR was significantly higher at 1.5T (P < .0001). Noteworthy, both observers noted a higher signal in the outer margins than that in the core of the lesion on FLAIR sequence at 3T imaging, providing an excellent contrast between the lesion boundaries and the apparently normal adjacent brain tissue.
Fig. 2.
Comparison between 1.5 and 3T. A. Graph showing linear regression (black line) with 95% CI (dotted lines) of lesion volumes at 1.5-T and 3-T (rspearman = 0.96 [95% CI:0.92−0.98]) Each dot represents a patient. B. Graph showing linear regression (black line) with 95% CI (dotted lines) of rCBV values on PWI at 1.5T and 3T (rspearman = 0.90 [95% CI:0.79−0.96]).
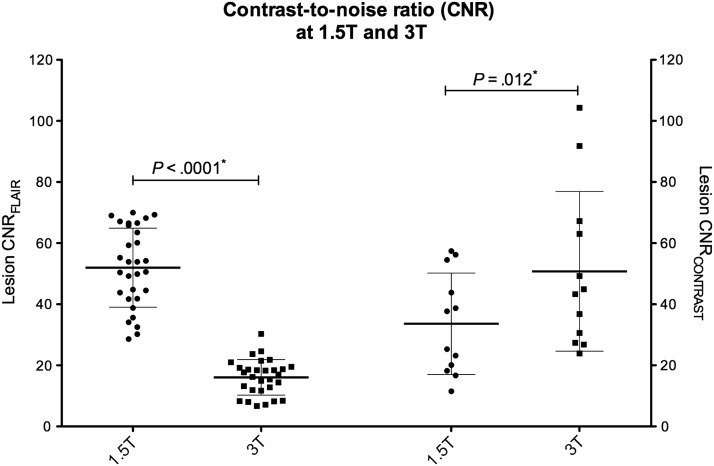
CNRCONTRAST was significantly higher at 3T (P = .012) (Fig. 3). The same number of areas of focal contrast enhancement was detected on 1.5 and 3T (Table 2), with only slight differences in classification (wκ = 0.96 (95% CI, 0.83–1). Perfusion parameters were available for 1.5 and 3T modalities for 25 (83%) participants. Although most rCBV values were low (mean rCBV1.5T = 0.99 ± 0.69 and rCBV3T = 1.10 ± 1.04, respectively; P = .29), correlation between modalities was excellent (ICC = 0.92 [95% CI, 0.85–0.97]) (Fig. 2B).
Fig. 3.
Contrast-to-noise ratio. Scatter-plot comparing lesion CNR on FLAIR sequences (CNRFLAIR) and lesion enhancement on 3D T1-w images after injection of contrast medium (CNRCONTRAST), using Wilcoxon matched-pairs signed-rank test (P*).
Impact on Response Assessment According to Response Assessment in Neuro-Oncology Criteria
Participants with WHO grade II gliomas (n = 26) were evaluated using RANO criteria for diffuse low-grade gliomas,1 and participants with WHO grade III gliomas (n = 4) were evaluated using RANO criteria for high-grade gliomas.2 At the time of the index MRIs, 4 participants (13%) were considered as having progressive disease in comparison with the previous evaluation. Five additional participants (17%) had progressed 3 to 6 months after the index MRIs. All other participants were considered as having stable disease, and no complete, partial, or minor response was registered. Participant response assessment was the same using the 1.5 or the 3T index MRI.
Discussion
We show for the first time, in 30 WHO grade II and III supratentorial gliomas, that tumor morphological features (volume, contrast enhancement) are similar between 1.5T and 3T MRI. Furthermore, we found that interobserver and intraobserver reproducibility was excellent regardless of the MR field.
The design of our study allowed accurate comparisons between the 2 MRI modalities since the same participants were scanned at both 1.5T and 3T magnetic fields on a magnet from the same manufacturer, using similar head coils and acquisition times. These results may have important practical implications for patient care: (i) they suggest that the MR images obtained during follow-up of a WHO grade II or III glioma can be acquired and analyzed regardless of the MR field strength; (ii) they strengthen the reliability of comparing tumor evolution based on serial MRIs from MR units with different magnetic fields; (iii) they provide additional flexibility in scheduling the MR follow-up of such patients since referring centers are increasingly equipped with more than one MR unit; and (iv) they offer the possibility of performing MR follow-up in different institutions, given the multidisciplinary and/or multicenter approach for managing such gliomas. Beyond clinical care, the present results may also simplify image acquisition for assessment of tumor response following oncological treatment and outcomes in prospective trials.
The excellent volume matching we observed between 1.5 and 3T may seem surprising, given that lesion CNR was higher on FLAIR at 1.5T. However, the lower CNR at 3T within the lesion was likely compensated by the high signal of the outer margins of the lesion on 3T imaging, which provided excellent contrast with the apparently normal adjacent brain tissue. This contrast may be related to a higher cellular density at the peripheral part of the tumor than its core, a finding we have previously observed in large tumors using a correlative pathological and imaging analysis with cell quantification.20 Regarding contrast enhancement, CNR was significantly higher on 3T in comparison with 1.5T, which as in line with previous preclinical16,21 and clinical studies.11 This did not, however, translate into a higher detection rate of focal contrast enhancement in our population. Nevertheless, the higher CNR we observed on 3T postcontrast images may allow a reduction in the quantity of contrast medium at 3T.11
We further found that rCBV values were consistent between 1.5 and 3T, whereas higher rCBV values at 3T in comparison with 1.5T have been reported in a small series of intra-axial space-occupying lesions.22 These differences may, at least in part, be related to differences in the PWI sequence parameters between the 1.5 and 3T MRI used in the latter study, whereas our parameters were kept almost identical between the 2 MR fields. Our results should, however, be interpreted with caution given that most participants had rCBV values well below the 1.7523 cut-off at both 1.5 and 3T and therefore did not encompass the whole spectrum of brain gliomas, particularly glioblastomas. We cannot exclude that a “bottom effect” could have masked potential differences between rCBV values obtained at 1.5T and 3T.
The present study has several limitations: (i) although the database was prospectively maintained and participants consecutively enrolled, the analysis was retrospective; (ii) the study sample is small but represents the only study comparing MR images of WHO grades II and III gliomas scanned on 1.5 and 3T magnetic fields in a short period of time; (iii) the present findings must be extrapolated with caution to MR units from other manufacturers because sequence parameters and image quality vary between machines, which could introduce a potential factor for variability; (iv) the acquisition times were short and similar on both MR field strengths. Longer acquisition times would improve image quality on 1.5 or 3T, and differences might then become apparent; (v) given the potential additional variability that could arise with less-experienced radiologists, these results should be extrapolated with caution to readers who are not specialized in neuro-oncology; (vi) readers were not blinded to magnetic field strength given that images are visually distinguishable, a limitation inherent to all studies comparing 1.5T and 31.5T images; and (vii) we did not compare spectroscopy, diffusion-weighted imaging, or perfusion parameters such as permeability, which may differ between 1.5 and 3T.
In this study we have shown that 1.5T and 3T units provided similar MR features in participants with WHO grades II and III gliomas with high interobserver and intraobserver reproducibility, suggesting that such gliomas can be imaged with comparable results using either magnetic fields.
Funding
No funding grant was received for this study.
Conflict of interest statement. None declared.
Acknowledgments
The authors would like to thank Stephanie Lion for supervising participant inclusions and imaging protocol implementation and Philippe Page for participant clinical follow-up.
References
- 1.van den Bent MJ, Wefel JS, Schiff D, et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12(6):583–593. [DOI] [PubMed] [Google Scholar]
- 2.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 3.Vogelbaum MA, Jost S, Aghi MK, et al. Application of novel response/progression measures for surgically delivered therapies for gliomas: Response Assessment in Neuro-Oncology (RANO) Working Group. Neurosurgery. 2012;70(1):234–243; discussion 243–234. [DOI] [PubMed] [Google Scholar]
- 4.Mandonnet E, Delattre JY, Tanguy ML, et al. Continuous growth of mean tumor diameter in a subset of grade II gliomas. Ann Neurol. 2003;53(4):524–528. [DOI] [PubMed] [Google Scholar]
- 5.Brasil Caseiras G, Ciccarelli O, Altmann DR, et al. Low-grade gliomas: six-month tumor growth predicts patient outcome better than admission tumor volume, relative cerebral blood volume, and apparent diffusion coefficient. Radiology. 2009;253(2):505–512. [DOI] [PubMed] [Google Scholar]
- 6.Rees J, Watt H, Jager HR, et al. Volumes and growth rates of untreated adult low-grade gliomas indicate risk of early malignant transformation. Eur J Radiol. 2009;72(1):54–64. [DOI] [PubMed] [Google Scholar]
- 7.Pallud J, Capelle L, Taillandier L, et al. Prognostic significance of imaging contrast enhancement for WHO grade II gliomas. Neuro Oncol. 2009;11(2):176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pallud J, Mandonnet E, Duffau H, et al. Prognostic value of initial magnetic resonance imaging growth rates for World Health Organization grade II gliomas. Ann Neurol. 2006;60(3):380–383. [DOI] [PubMed] [Google Scholar]
- 9.Pallud J, Blonski M, Mandonnet E, et al. Velocity of tumor spontaneous expansion predicts long-term outcomes for diffuse low-grade gliomas. Neuro Oncol. 2013;15(5):595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sasaki M, Inoue T, Tohyama K, et al. High-field MRI of the central nervous system: current approaches to clinical and microscopic imaging. Magn Reson Med Sci. 2003;2(3):133–139. [DOI] [PubMed] [Google Scholar]
- 11.Krautmacher C, Willinek WA, Tschampa HJ, et al. Brain tumors: full- and half-dose contrast-enhanced MR imaging at 3.0T compared with 1.5 T--Initial Experience. Radiology. 2005;237(3):1014–1019. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen K, Rostrup E, Frederiksen JL, et al. Magnetic resonance imaging at 3.0 tesla detects more lesions in acute optic neuritis than at 1.5 tesla. Invest Radiol. 2006;41(2):76–82. [DOI] [PubMed] [Google Scholar]
- 13.Mellerio C, Labeyrie MA, Chassoux F, et al. 3T MRI improves the detection of transmantle sign in type 2 focal cortical dysplasia. Epilepsia. 2014;55(1):117–122. [DOI] [PubMed] [Google Scholar]
- 14.Dhermain FG, Hau P, Lanfermann H, et al. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol. 2010;9(9):906–920. [DOI] [PubMed] [Google Scholar]
- 15.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biswas J, Nelson CB, Runge VM, et al. Brain tumor enhancement in magnetic resonance imaging: comparison of signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) at 1.5 versus 3 tesla. Invest Radiol. 2005;40(12):792–797. [DOI] [PubMed] [Google Scholar]
- 17.Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates and Proportions. Hoboken, New Jersey: John Wiley & Sons; 2013. [Google Scholar]
- 18.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86(2):420–428. [DOI] [PubMed] [Google Scholar]
- 19.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 20.Gerin C, Pallud J, Deroulers C, et al. Quantitative characterization of the imaging limits of diffuse low-grade oligodendrogliomas. Neuro Oncol. 2013;15(10):1379–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sasaki M, Shibata E, Kanbara Y, et al. Enhancement effects and relaxivities of gadolinium-DTPA at 1.5 versus 3 Tesla: a phantom study. Magn Reson Med Sci. 2005;4(3):145–149. [DOI] [PubMed] [Google Scholar]
- 22.Mauz N, Krainik A, Tropres I, et al. Perfusion magnetic resonance imaging: comparison of semiologic characteristics in first-pass perfusion of brain tumors at 1.5 and 3 Tesla. J Neuroradiol. 2012;39(5):308–316. [DOI] [PubMed] [Google Scholar]
- 23.Caseiras GB, Chheang S, Babb J, et al. Relative cerebral blood volume measurements of low-grade gliomas predict patient outcome in a multi-institution setting. Eur J Radiol. 2010;73(2):215–220. [DOI] [PubMed] [Google Scholar]