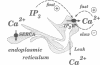
Abstract
Combining a realistic model of inositol 1,4,5-trisphosphate (IP3)-induced Ca2+ oscillations with the diffusion of IP3 and buffered diffusion of Ca2+, we have found that diffusion of Ca2+ plays only a minor role in a class of agonist-induced Ca2+ wave trains. These waves are primarily kinematic in nature, with variable wavelengths and speeds that depend primarily on the phase differences between oscillators at different spatial points. The period is set by the steady-state value of IP3, while the wave speed approximately equals the wavelength/period. Ca2+ diffusion, which is much slower than that of IP3 because of endogenous buffers, is shown to have only a small effect on the wave trains and not to be necessary for the apparent wave propagation. Diffusion of IP3 sets the phase gradient responsible for these wave trains, which consist primarily of localized cycles of Ca2+ uptake and release. Our results imply a possible previously undisclosed role for IP3 in cell signaling.
Full text
PDF
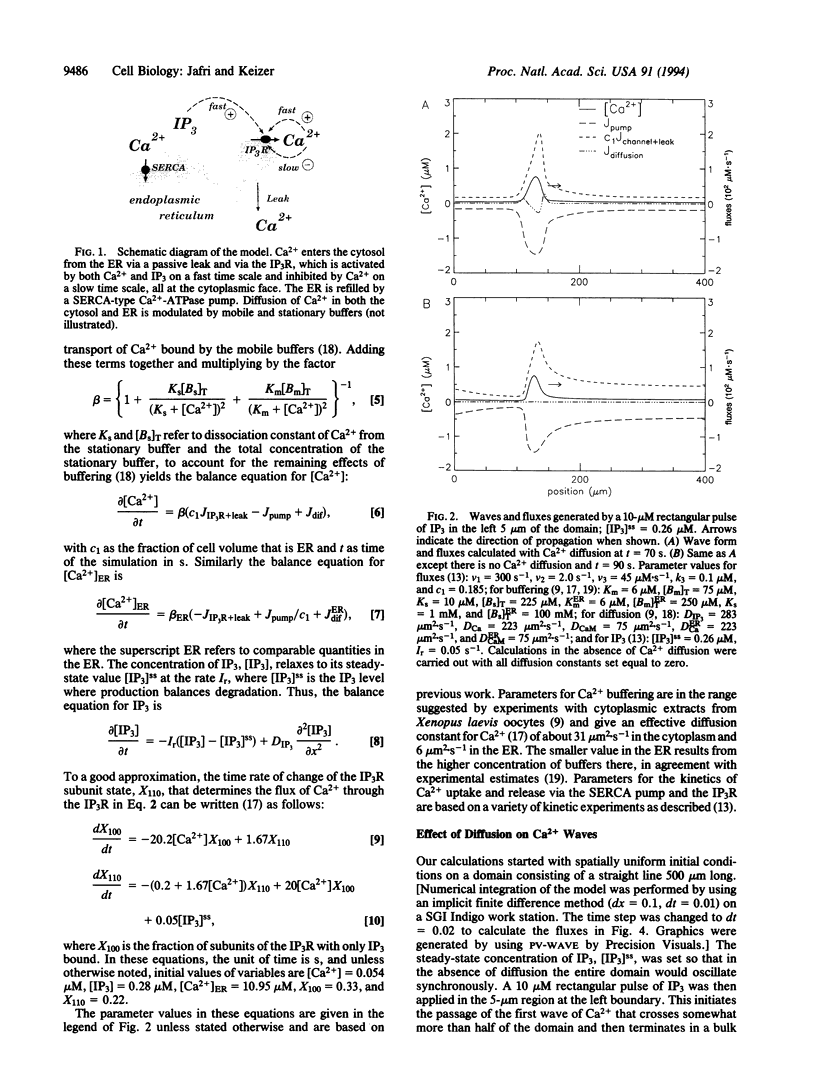

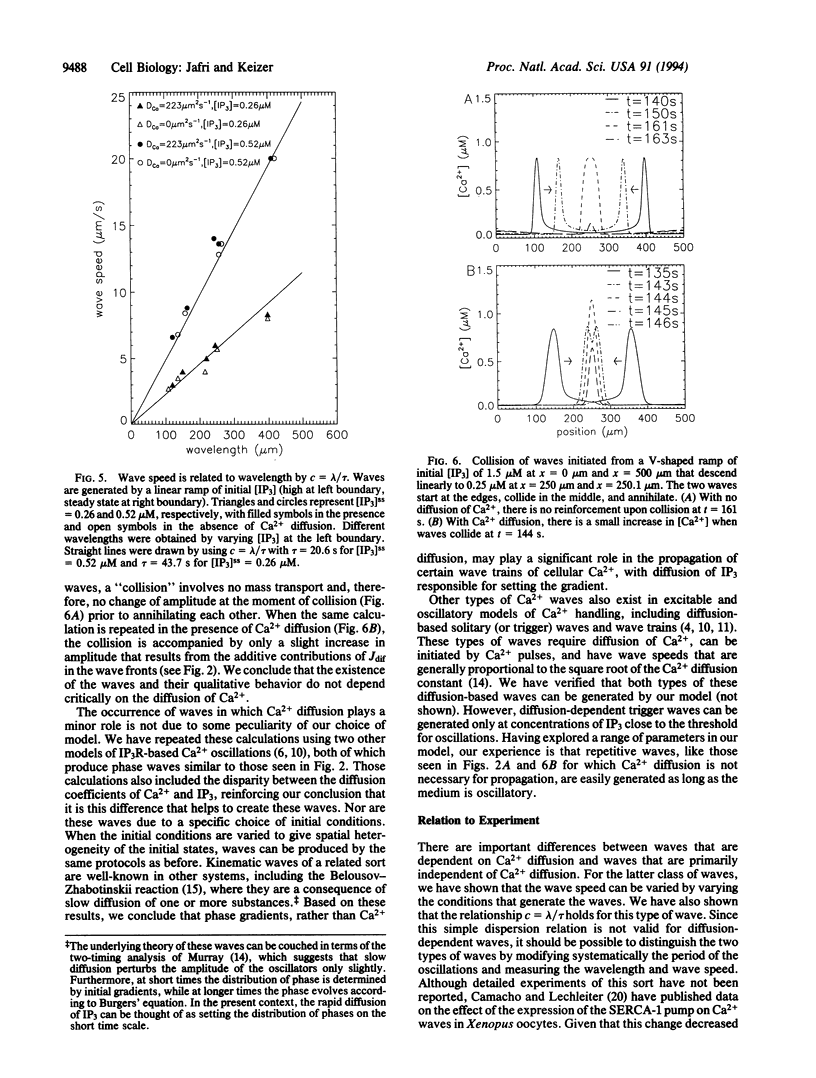
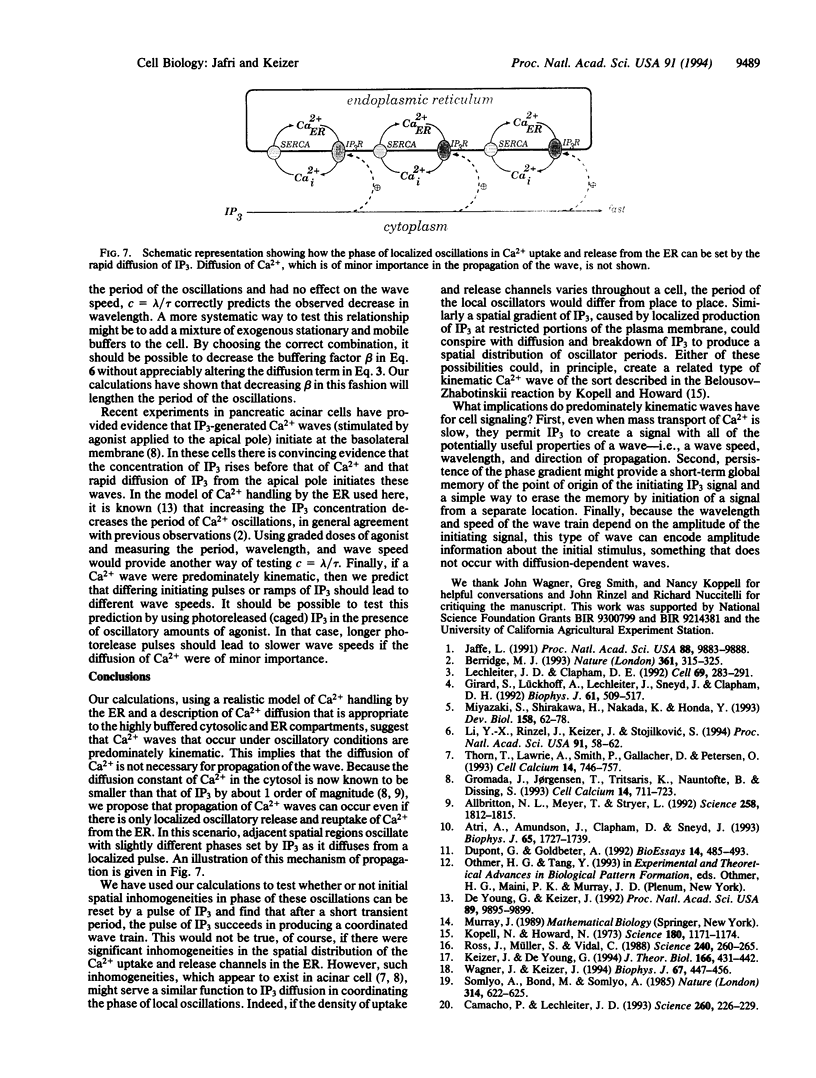
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allbritton N. L., Meyer T., Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992 Dec 11;258(5089):1812–1815. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
- Atri A., Amundson J., Clapham D., Sneyd J. A single-pool model for intracellular calcium oscillations and waves in the Xenopus laevis oocyte. Biophys J. 1993 Oct;65(4):1727–1739. doi: 10.1016/S0006-3495(93)81191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Camacho P., Lechleiter J. D. Increased frequency of calcium waves in Xenopus laevis oocytes that express a calcium-ATPase. Science. 1993 Apr 9;260(5105):226–229. doi: 10.1126/science.8385800. [DOI] [PubMed] [Google Scholar]
- De Young G. W., Keizer J. A single-pool inositol 1,4,5-trisphosphate-receptor-based model for agonist-stimulated oscillations in Ca2+ concentration. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9895–9899. doi: 10.1073/pnas.89.20.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont G., Goldbeter A. Oscillations and waves of cytosolic calcium: insights from theoretical models. Bioessays. 1992 Jul;14(7):485–493. doi: 10.1002/bies.950140711. [DOI] [PubMed] [Google Scholar]
- Girard S., Lückhoff A., Lechleiter J., Sneyd J., Clapham D. Two-dimensional model of calcium waves reproduces the patterns observed in Xenopus oocytes. Biophys J. 1992 Feb;61(2):509–517. doi: 10.1016/S0006-3495(92)81855-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromada J., Jørgensen T. D., Tritsaris K., Nauntofte B., Dissing S. Ca2+ signalling in exocrine acinar cells: the diffusional properties of cellular inositol 1,4,5-trisphosphate and its role in the release of Ca2+. Cell Calcium. 1993 Nov;14(10):711–723. doi: 10.1016/0143-4160(93)90097-p. [DOI] [PubMed] [Google Scholar]
- Jaffe L. F. The path of calcium in cytosolic calcium oscillations: a unifying hypothesis. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9883–9887. doi: 10.1073/pnas.88.21.9883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopell N., Howard L. N. Horizontal bands in the belousov reaction. Science. 1973 Jun 15;180(4091):1171–1173. doi: 10.1126/science.180.4091.1171. [DOI] [PubMed] [Google Scholar]
- Lechleiter J. D., Clapham D. E. Molecular mechanisms of intracellular calcium excitability in X. laevis oocytes. Cell. 1992 Apr 17;69(2):283–294. doi: 10.1016/0092-8674(92)90409-6. [DOI] [PubMed] [Google Scholar]
- Li Y. X., Rinzel J., Keizer J., Stojilković S. S. Calcium oscillations in pituitary gonadotrophs: comparison of experiment and theory. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):58–62. doi: 10.1073/pnas.91.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S., Shirakawa H., Nakada K., Honda Y. Essential role of the inositol 1,4,5-trisphosphate receptor/Ca2+ release channel in Ca2+ waves and Ca2+ oscillations at fertilization of mammalian eggs. Dev Biol. 1993 Jul;158(1):62–78. doi: 10.1006/dbio.1993.1168. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Bond M., Somlyo A. V. Calcium content of mitochondria and endoplasmic reticulum in liver frozen rapidly in vivo. Nature. 1985 Apr 18;314(6012):622–625. doi: 10.1038/314622a0. [DOI] [PubMed] [Google Scholar]
- Thorn P., Lawrie A. M., Smith P. M., Gallacher D. V., Petersen O. H. Ca2+ oscillations in pancreatic acinar cells: spatiotemporal relationships and functional implications. Cell Calcium. 1993 Nov;14(10):746–757. doi: 10.1016/0143-4160(93)90100-k. [DOI] [PubMed] [Google Scholar]
- Wagner J., Keizer J. Effects of rapid buffers on Ca2+ diffusion and Ca2+ oscillations. Biophys J. 1994 Jul;67(1):447–456. doi: 10.1016/S0006-3495(94)80500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]