Abstract
Slightly focused high-energy shockwave (HESW) therapy is characterized by a wide focal area, a large therapy zone, easy positioning and less pain during treatment. The objective of this study was to perform for the first time an in vivo test of the slightly focused HESWs for osteoporotic fractures. Bilateral proximal tibial osteotomies were made in 30 ovariectomized (OVX) Sprague-Dawley rats and secured with internal fixation. The osteotomy site in the left tibia was subsequently treated with slightly focused HESWs with the energy flux density of 0.26 mj/mm2, shock repetition frequency of 1 Hz and 2000 shocks (OVX + HESW group). The contralateral right tibia was not treated and served as the control (OVX group). Roentgenographic examination 2, 4, 6, and 8 weeks after osteotomy showed that HESW treatment accelerated tibia fracture healing in osteoporotic rats. Histological examination 2, 4, and 8 weeks after HESW treatment showed a greater inflammatory reaction in the OVX + HESW group, with more mature collagen and trabeculae than in the OVX group. Micro computer tomography (Micro-CT) scanning after 4 and 8 weeks showed that bone volume (BV), bone volume/tissue volume (BV/TV), mean trabecular thickness (Tb.Th), and mean trabecular number (Tb.N) were about 45.0% and 33.1%, 18.4% and 20.1%, 38.2% and 20.9%, 26.7% and 28.4%, respectively, higher in the treatment group than in the control group (P < 0.05); and the mean trabecular separation (Tb.Sp) was about 16.7% and 27.3% lower in the treatment group (P < 0.05). Four and eight weeks after HESW treatment, the maximum compressive callus endurance was about 72.3% and 25.5%, respectively, higher in the treatment group than in the control group (P < 0.05). These results show that slightly focused HESW therapy has a beneficial effect on osteoporotic tibial fracture healing. Slightly focused HESWs could increase callus endurance, induce bone formation, and improve trabecular bone microarchitecture and biomechanical properties.
Keywords: High-energy shockwave, osteoporotic fracture, fracture healing, bone microstructure, biomechanical properties
Introduction
Osteoporosis is defined as a systemic disease characterized by the loss of bone mass and deterioration of bone microarchitecture with consequent increases in bone fragility and susceptibility to fracture [1]. Postmenopausal osteoporosis is frequently caused by the combination of low bone mass and menopausal bone loss [1-3]. Age is an additional major risk factor for osteoporosis [2,3]. As a health-care problem, osteoporosis currently accounts for a large amount of both public and private health spending, and its prevalence is expected to escalate rapidly in the 21st century, with the estimated cost for treating hip fractures alone reaching 131 billion United States Dollar (USD) worldwide by 2050 [4-6].
Fracture is the ultimate and most catastrophic consequence of osteoporosis. The relationship between bone fracture healing and osteoporosis is complex [7,8]. Although much attention has been paid to fracture prevention and new therapies aimed at conserving bone mass, little emphasis has been given to the study of fracture healing in osteoporotic bone [9]. In patients with osteoporosis, fracture healing is delayed, and the recovery period is longer [7-9]. Moreover, after fracture healing, osteoporosis is ultimately aggravated, and the risk of refracture is significantly greater than in healthy bone. When treating an osteoporotic fracture with open reduction and internal fixation, surgeons should be aware that this treatment may result in a reduction in bone mass and that refracture may occur.
In the past, long-term medications were needed to treat osteoporotic patients in order to avoid refracture, meaning the medical treatment period was long with high costs and thus poor compliance in elderly patients [10,11]. Recently, High-energy shockwave (HESW) therapy has been identified as an effective noninvasive therapy for stimulating bone healing in selected patients with musculoskeletal diseases such as nonunion, avascular necrosis of the femoral head and tennis elbow [12-16]. HESWs are high-energy single sonic pulses generated underwater by high-voltage explosion and vaporization, which propagate in a wavelike manner in water-like soft tissues with minimal tissue absorption and no thermal effect [17]. HESWs are characterized by a high peak pressure (100 MPa) with an energy flux density in the range of 0.003-0.890 mJ/mm2 [18]. When the pressure waves meet an interface of different impedance in their flow, the energy will be released to generate shear forces and cavitation, which could cause microcracks in bone tissue as well as subperiosteal hemorrhage, micro-scale damage of bone trabeculae, and minor hemorrhaging within the medullary cavity [19,20], thereby inducing multi-biological effects such as the stimulating callus growth, inducing vascular regeneration [21], promoting bone formation, and relieving pain [22].
The effect of shock waves on bone healing has been a matter of debate among researchers [23-27]. Thus, although some authors postulated that shockwave treatment might delay bone fracture healing [23], others showed that HESWs could promote osteogenesis, improve bone structure and quality, and increase bone mineral density (BMD) [14,24-29]. HESW therapy is increasingly used as an adjuvant treatment for fresh fractures [30,31].
However, there is no agreement regarding the mechanism of HESW action on the healing of osteoporotic fractures. It remains unclear whether HESWs could induce osteoblasts or restrain osteoclasts and whether they could improve bone healing and biomechanical properties in the osteoporotic fracture healing process. A new generation of slightly focused HESW therapy has wider focal area, larger therapy zone, easier positioning and less pain during treatment, which is more suitable for application in orthopedics [11]. There are not yet sufficient data to allow a direct comparison of focused and slightly focused HESW therapies for a particular clinical application [32], in other words, no evidence in terms of outcome clearly favors one type of HESW over the other. However, application of the focused one to select locations may be prevented because of the traditionally larger heads of devices [32]; and piezoelectric-, electromagnetic- and electrohydraulic-generated shock waves can be administered only be trained physicians [32]. The aim of this experimental study was to perform for the first time an in vivo test of the slightly focused HESWs for osteoporotic fractures and to investigate whether slightly focused HESWs could induce new bone formation and improve both bone microarchitecture and mechanical properties in a rat model of osteoporotic fracture.
Materials and methods
Animals
For this experimental animal study, 42 female Sprague-Dawley (S-D), clean-level rats, aged 3 months, with an average weight (± standard deviation [SD]) of 200 ± 2.5 g (Xi Puer-Bi kai, Shanghai, China) were used. This research was approved by the animal welfare ethics committee of the 6th People’s Hospital affiliated with Shanghai Jiaotong University (permission number: SCXK, Shanghai 2008-0016).
Experiment design
Induction of osteoporosis
Ovariectomized (OVX) rats have been widely used as a model for postmenopausal osteoporosis and validated as a clinically relevant model of human postmenopausal bone loss [33,34]. The S-D rats were divided into a sham-operated group (n = 6) and an operated group (n = 36). The rats of the operated group underwent open bilateral oophorectomy and ligation of the oviduct and comites. In contrast, for rats in the sham-operated group, the ovaries were anatomically found and exposed, and then the surgical wound was closed. Postoperatively, all 42 rats were housed in a cage with 12-h light/dark conditions at 21°C and 60% humidity.
After conventional breeding for 3 months, 6 randomly selected rats from the operated group and the sham-operated group were sacrificed. Whole body was scanned and BMDs were measured at the L5 vertebrae, femoral neck, and proximal tibiae using dual-energy X-ray absorptiometry (DEXA, owning rat BMD measurement software, Hologic Delphi, America). The exposure conditions were the energy spectrum of 38/70 keV, high voltage stability of ± 0.05% and precision of 1%. Osteoporosis in the operated rats was defined as a BMD (L5 and/or femoral neck and/or proximal tibiae) more than 2.5 SD below the mean BMD acquired from the sham-operated group [8]. BMDs at L5 and femoral neck in operated group were more than 2.5 SD below the mean BMD in the sham-operated group (Table 1, P < 0.001); and BMD at proximal tibiae in the operated group was 12.9% lower than that in the sham-operated group (Table 1, P < 0.05).
Table 1.
Comparison of the absolute values of BMD (g/cm2) measured at three different locations (Proximal tibia, L5, and Femoral neck)
| Group\Location | Proximal tibia | L5 | Femoral neck |
|---|---|---|---|
| Sham-operated group (n = 6) | 0.928 ± 0.072 | 0.271 ± 0.006 | 0.452 ± 0.021 |
| Operated group (n = 6) | 0.808 ± 0.09 | 0.236 ± 0.005 | 0.356 ± 0.017 |
| T value | -2.550 | -10.977 | -8.703 |
| P value | < 0.05 | < 0.001 | < 0.001 |
BMD values were expressed as mean (g/cm2) ± SD, standard deviation. The P values were obtained using Student’s t-test. P value < 0.05 was considered statistically significant.
Tibia fracture creation and internal fixation
Following induction of osteoporosis in the remaining operated rats, a bilateral proximal incomplete (1/3 of cross-sectional area) tibial osteotomy was created using an oscillating saw and subsequently stabilized with intramedullary pins [35-37]. The accuracy of proximal tibial osteotomy was documented radiographically. Then the established experimental model of osteoporotic fracture was applied in further experiments.
Application of slightly focused HESWs
One week after internal fixation, the middle part of the osteotomy site on the left tibia was marked with methyl violet and treated once with HESWs using OrthospecTM (Medispec, Yehud, Israel). The focal diameter and length were 25 mm and 95 mm, respectively. The exposure conditions were the energy flux density of 0.26 mj/mm2, shock repetition frequency of 1 Hz, and 2000 shocks [35-37]. The left tibia was set as the treatment side (treated group: OVX + HESW), whereas the contralateral right tibial osteotomy served as the control side (control group: OVX) and was not treated with HESWs. After HESW treatment, conventional rat breeding continued. Callus formation in the rats of both groups was regularly investigated roentgenographically, histologically, using micro-CT scanning, and biomechanically, as detailed in the following sections.
Test items
Radiographic evaluation of callus formation
X-ray images of both tibiae in the S-D rats were obtained 2, 4, 6, and 8 weeks after osteotomy to confirm adequate callus formation and fracture healing.
Callus histomorphometry
Six randomly selected S-D rats were killed at 2, 4, and 8 weeks after HESW treatment. One-centimeter longitudinal callus tissues were taken from the middle part of the tibial osteotomy sites. The tissues were fixed with 4% paraformaldehyde solution immediately after rinsed with Phosphate Buffered Saline. Subsequently, the tissues were decalcified, dehydrated, allowed to become transparent, longitudinally paraffin embedded, cut into 7-µm serial sections using a hard tissue slicing machine (LEICA SP1600 and SW2500, Wetzlar, Germany), and stained with hematoxylin and eosin (H&E). The slices were examined under light microscopy (LEICA, DM4000B).
Micro-CT measurement
Six S-D rats were randomly sacrificed 4 and 8 weeks after HESW treatment. Muscles and soft tissue were removed, while the periosteum was kept intact to the extent possible. Kirschner pins were removed, and a 2-cm long bone segment, including the fracture callus, was selected subjected to micro-CT (Skyscan1076, Belgium). The acquisition settings were the X-ray source voltage of 80 kV and current of 450 μA. The rotation step and angular step (deg) was 0.6°, with a complete rotation over 360°; the image pixel size was 18 μm. Parameters such as tissue volume (TV), bone volume (BV), bone volume/tissue volume (BV/TV), bone surface (BS), bone surface/bone volume (BS/BV), bone surface/tissue volume (BS/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N) and trabecular separation (Tb.Sp) were measured respectively.
Biomechanical testing
In the three-point bending test, the loading bar was placed at the fracture site to test the specific part of the bone [38,39]. The tibial specimens were removed from deep freezing and thawed under normal temperature. The specimens were subsequently placed in a Zwick/Roell testing machine (BZ2.5/TSIS, Germany) for a three-point bending test. The bearing span was 20 mm. The middle of the tibial osteotomy site was set as the loading point with a loading speed of 1 mm/min (Figure 1). During the loading process, the loading direction was consistent and the specimens were kept wet. The real-time test process was monitored by computer, which could automatically draw every measured value of samples and record the load-displacement curve and maximum load.
Figure 1.

The three-point bending test on the tibia. The bearing span was 20 mm. The middle of the tibial osteotomy was set as the loading point with a loading speed of 1 mm/min. During the loading process, the loading direction was consistent and the specimens were kept wet.
Statistical analysis
All studied parameters were tested normatively. Descriptive statistics included means and SDs for continuous variables, and frequencies and percentages for categorical variables. Means of parametric values were compared between groups using t-tests and of non-parametric values with chi-squared tests. P-values smaller than 0.05 were considered statistically significant.
Results
Radiographic evaluation of callus formation
Two weeks after internal fixation, tibial callus growth was beginning in both groups (Figure 2A2, 2B2). At 4 weeks, the fracture gap began to decrease and the callus volume was significantly reduced (Figure 2A3, 2B3). Callus dimensions in the treatment group were larger than in the control group at 2 and 4 weeks after surgery. At 6 weeks, most rats of the treatment group had almost achieved osseous healing, whereas partial fracture lines were still seen in the control group (Figure 2A4, 2B4). At 8 weeks, osseous healing had occurred in the rat tibiae of both groups, whereas the repair of fracture and the reconstruction of bone at the tibial osteotomy site in the treatment group were faster (Figure 2A5, 2B5).
Figure 2.
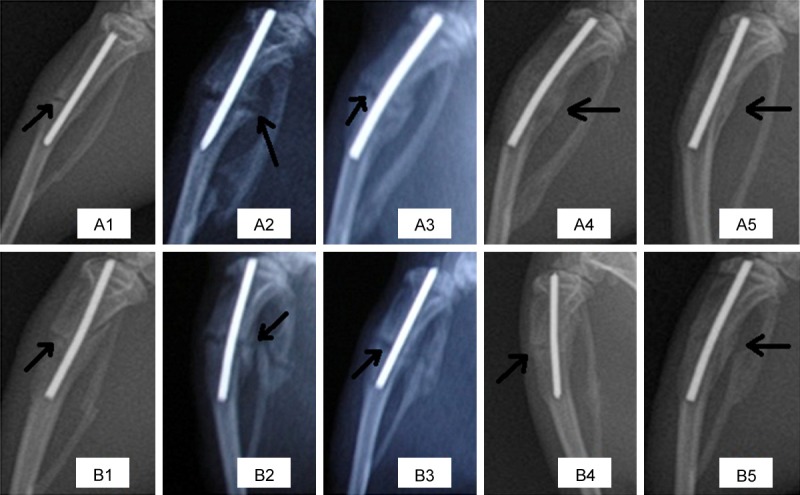
X-ray images of fracture fixation post-operation in the OVX + HESW (A1-A5) and OVX (B1-B5) groups on the day of fixation and 2, 4, 6 and 8 weeks later. At 2 weeks (A2, B2), callus growth could be clearly seen in both groups. At 4 weeks (A3, B3), callus formation through the fracture line could be clearly seen in both groups, and the gap at the fracture end of the treatment group had decreased. At 6 weeks (A4, B4), the treatment group basically had achieved osseous healing, whereas the fracture end gap still could be seen in the control group. At 8 weeks (A5, B5), the rats of both groups had achieved osseous healing.
Callus histomorphometry
Histological examination showed that the fibrous callus in both groups was gradually replaced by cartilaginous callus 2 weeks after HESW therapy, and there was a small amount of lamellar bone trabeculae. Compared with the control group, in the treatment group more superficial trabeculae, collagen formation, and aligned osteoblasts were observed (Figure 3A1, 3B1). At 4 weeks, the peripheral part of the cartilaginous callus was continuously replaced by primitive trabeculae (woven bone). The chondrocytes within the original bone trabeculae had degenerated or transformed to island soft cartilage, which was less in the control group. In the treatment group, more mature trabeculae and osteoid tissue were observed within the retrieved specimen than in the control group in which the trabeculae were fewer in number, with slow cartilage ossification (Figure 3A2, 3B2). Uneven thickness and disorganized trabecular bone were inconsistent with the stress direction. At the periphery of trabeculae, the absolute numbers of osteoblasts and osteocytes on the control side were less than those in the treatment group. At 8 weeks, the process of bone remodeling had been completed. More cortical bone had been generated in the treatment group, whereas on the control side, the trabeculae were still in the reconstruction phase (Figure 3A3, 3B3).
Figure 3.
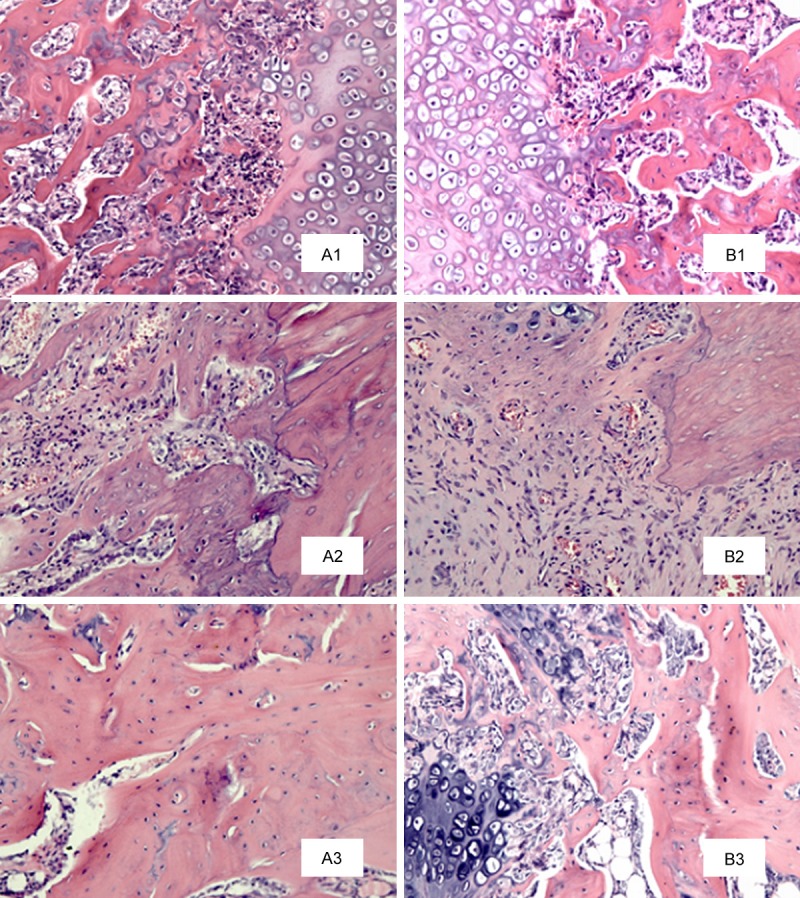
H&E staining of the microscopic structure of the fracture end in the OVX + HESW (A1-A3) and OVX (B1-B3) groups at 2, 4 and 8 weeks after slightly focused HESW treatment. Collagen density of the control group was low at 2 weeks (B1), and less mature collagen of bone trabecula was observed. The inflammatory reaction in the treatment group was stronger, and relatively more mature collagen and new trabecula were observed (A1). At 4 weeks (A2, B2), more mature trabecula of treatment group was observed, and the cartilaginous callus was gradually replaced by bony callus with a large amount of osteoid tissue, which was more in the treatment group. At 8 weeks (A3, B3), reconstruction of trabeculae of the control group, which continued to show a disordered arrangement (H&E; magnification, × 200).
Micro-CT measurement
At 4 and 8 weeks after HESW treatment, bone volume (BV), bone volume/tissue volume (BV/TV), average trabecular thickness (Tb.Th), and average number of trabeculae (Tb.N) in the left (treated) tibial specimens were about 45.0% and 33.1%, 18.4% and 20.1%, 38.2% and 20.9%, 26.7% and 28.4%, respectively, higher (P < 0.05) than in the untreated control side (Table 2). And compared to that in the control group, the mean trabecular separation (Tb.Sp) in the treatment group was 16.7% and 27.3%, respectively, lower (Table 2, P < 0.05). Micro-CT scanning and three-dimensional reconstruction showed that callus growth and bone microstructure were superior in the treatment group (Figures 4, 5).
Table 2.
The parameters of the bone microarchitecture of rat tibia specimens (mean ± SD)
| 4 weeks (n = 6) | 8 weeks (n = 6) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| OVX + HESW | OVX | T value | P value | OVX + HESW | OVX | T value | P value | |
| TV (mm3) | 39.34 ± 3.24 | 31.15 ± 2.67 | -4.78 | < 0.001 | 29.10 ± 2.50 | 25.23 ± 1.98 | -2.97 | < 0.05 |
| BV (mm3) | 20.72 ± 4.13 | 14.29 ± 3.46 | -2.92 | < 0.05 | 16.20 ± 3.20 | 12.17 ± 2.37 | -2.48 | < 0.05 |
| BV/TV (%) | 51.70 ± 4.62 | 43.68 ± 5.10 | -2.85 | < 0.05 | 54.35 ± 3.14 | 45.24 ± 3.75 | -4.56 | < 0.005 |
| BS (mm2) | 189.87 ± 32.83 | 142.32 ± 29.46 | -2.64 | < 0.05 | 157.38 ± 24.50 | 110.72 ± 18.76 | -3.71 | < 0.005 |
| BS/BV (1/mm) | 9.06 ± 0.15 | 8.67 ± 0.08 | -5.62 | < 0.001 | 9.53 ± 0.12 | 8.74 ± 0.17 | -9.30 | < 0.001 |
| BS/TV (1/mm) | 4.83 ± 0.18 | 4.26 ± 0.12 | -6.45 | < 0.001 | 5.16 ± 0.23 | 4.38 ± 0.16 | -6.82 | < 0.001 |
| Tb.Th (mm) | 0.47 ± 0.05 | 0.34 ± 0.06 | -4.08 | < 0.005 | 0.52 ± 0.06 | 0.43 ± 0.04 | -2.82 | < 0.05 |
| Tb.N (N/mm) | 1.42 ± 0.18 | 1.12 ± 0.21 | -2.66 | < 0.05 | 1.31 ± 0.17 | 1.02 ± 0.23 | -2.48 | < 0.05 |
| Tb.Sp (mm) | 0.35 ± 0.04 | 0.42 ± 0.05 | 6.50 | < 0.001 | 0.32 ± 0.05 | 0.44 ± 0.03 | 5.04 | < 0.001 |
Values of all parameters were expressed as mean ± SD, standard deviation. TV, tissue volume; BV, bone volume; BV/TV, bone volume/tissue volume; BS, bone surface; BS/BV, bone surface/bone volume; BS/TV, bone surface/tissue volume; Tb.Th, trabecular thickness; Tb.N, trabecular number; Tb.Sp, trabecular separation. The P values were obtained using Student’s t-test. P value < 0.05 was considered statistically significant.
Figure 4.
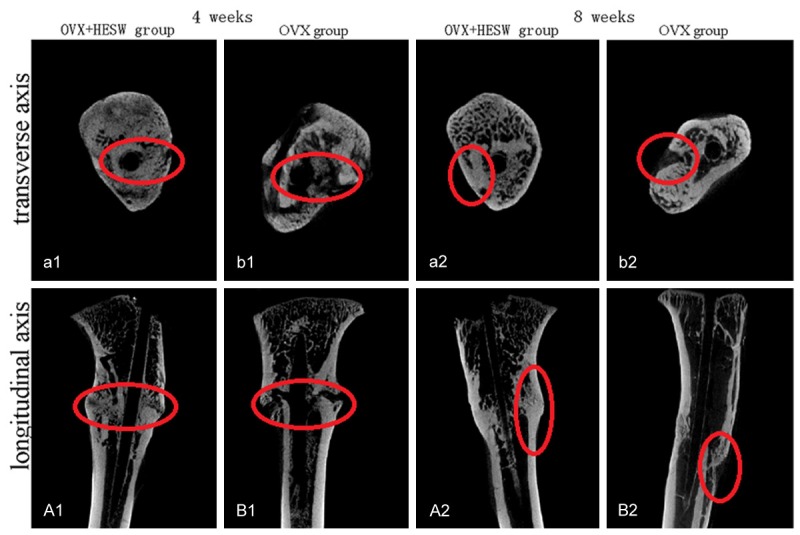
Micro-CT images of rat tibia in vitro in the OVX + HESW (a1, a2, A1, A2) and OVX (b1, b2, B1, B2) groups at 4 and 8 weeks after slightly focused HESW treatment. At 4 weeks, the callus quantity, thickness and continuity of the treatment group were significantly superior to those of the control group in images from Micro CT scanning along the longitudinal axis. The trabecula maturity and trabecular density of bone in the treatment group was better than that in the control group in images from Micro CT scanning along the transverse axis. At 8 weeks, fracture healing and bone reconstruction in the treatment group were better than in the control group.
Figure 5.
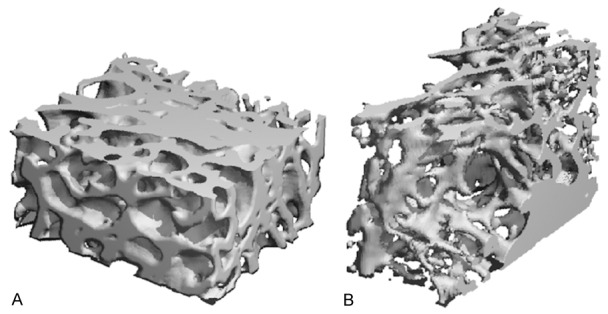
Three-dimensional reconstruction image of fractures at 8 weeks after slightly focused HESW treatment. A: In the treatment group (OVX + HESW), bone trabecular thickness was greater and arrangement was closer. B: In the control group (OVX), trabecular bone thickness was less and the space within was wider.
Biomechanical properties
All healed tibias osteotomed in both groups were broken at the fracture site. All tibias displayed a typical load-displacement curve with an initial nonlinear response followed by an upward-sloping linear component and then a failure response at the point of breakage (Figure 6). Compared with the control group, the maximum load endurance of the tibia was higher in the treatment group 4 and 8 weeks after HESW treatment (Table 3). At the osteotomy site, in the treatment group, the ultimate load to failure was increased by 72.3% and 25.5% at 4 and 8 weeks, respectively, after HESW treatment compared to that of the non-treated side.
Figure 6.
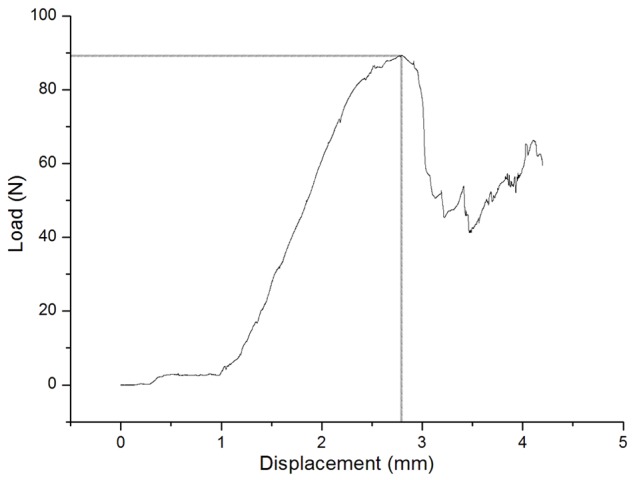
A typical load-displacement curve of OVX + HESW sample. The curve was characterized by an initial nonlinear response followed by an upward-sloping linear component and then a failure response at the point of breakage.
Table 3.
The maximum load of healing tibia in rats (unit: N)
| Groups | 4 weeks (n = 6) | 8 weeks (n = 6) |
|---|---|---|
| OVX | 27.48 ± 3.84 | 68.49 ± 6.62 |
| OVX + HESW | 47.34 ± 8.94 | 85.96 ± 4.25 |
| P value | < 0.001 | < 0.05 |
The maximum load values were expressed as mean (N) ± SD, standard deviation. The P values were obtained using Student’s t-test. P value of < 0.05 was used to determine statistical significance.
Discussion
In the past decade, osteoporosis has become a major public health concern. It is usually a clinically silent disease and, fairly often, an osteoporotic fracture comes up as its first manifestation. The fracture incidence in patients with osteoporosis is high [8,10,11]. With the increasing longevity and the growing size of the aging population, by 2050, more than 6 million hip fractures among elderly people will occur in the world [40].
After fixation of osteoporotic fractures, additional treatment may be also necessary. In the past, long-term medications were needed to avoid aggravated osteoporosis and refracture, which meant long medical treatment period, high costs and thus poor compliance in elderly patients [10,11]. Recently, HESW therapy has been increasingly used as an adjuvant treatment for musculoskeletal diseases such as fresh fracture, nonunion, avascular necrosis of the femoral head and tennis elbow [12-16,30,31], attributing to its multi-biological effects such as the stimulating callus growth, inducing vascular regeneration [21], promoting bone formation, and relieving pain [22]. HESWs have also been shown to stimulate large-scale inflammation and blood vessel reactions; increase the local blood supply; induce vascularization; enhance intramembranous ossification and endochondral bone formation; and promote fracture healing [41,42]. Slightly focused HESWs are characterized by wider focal area, larger therapy zone, easier positioning and less pain during treatment, which are more suitable for application in orthopedics [11]. In the present in vivo study, we aimed to investigate whether slightly focused HESWs had positive effects on healing process in a rat model of osteoporotic fracture.
Osteoporosis in humans is currently diagnosed with DEXA, which is considered as the gold standard for measuring BMD [43,44]. In this study, the BMD at the proximal tibia in the operated group was 12.9% less than that in the sham-operated group, revealing the influence of oophorectomy on bone mass reduction and osteoporosis induction in the early phase in a rat (Table 1). Three months after oophorectomy, the measured BMD changes in the operated rats confirmed osteoporosis (BMD < -2.5 SD; Table 1). So, this osteoporosis model was considered suitable in this in vivo study [8]. The experimental model of osteoporotic fracture in our study was established following a bilateral proximal incomplete tibial osteotomy using an oscillating saw and subsequently stabilized with intramedullary pins, which was characterized by simple operation, minor surgical wound and low infection and mortality [35-37]. In the present study, we aimed to investigate the effects of slightly focused HESWs on fresh osteoporotic fractures. Theoretically, the OVX + HESW group should be treated by HESWs as soon as the internal fixation was finished. Because of the surgical wound, however, more infection would occur if the rats were treated before the wound healing. So, one week after internal fixation might be the appropriate moment to receive HESW therapy [35,36].
In this study, after the internal fixation of an osteoporotic fracture, the radiological and histomorphometric results showed an increase in bridging callus formation during the early phase of fracture healing in the OVX + HESW group compared to the OVX group (Figures 2, 3). After fracture healing in both groups, the maximum compressive load of the left tibia after HESW treatment was about 25.5% higher than that of the right non-treated tibia, indicating that the biomechanical properties of the tibias in the treatment group were superior to those in the control group (Table 3). The results of radiographic evaluation, callus histomorphometry, and three-point bending test in the current study support the findings of Wang et al. [16,31] who showed that HESW therapy produces significantly greater bone mass (BMD, etc.), callus size, ash and calcium content, and bone strength compared to controls in femoral fractures in rabbits [16,31]. The results of the present study also indicated that the changes in the biomechanical performance of the healed bone were actually reflected by the radiological and histomorphological changes detected in the bone after HESW treatment.
Bone mass (e.g. BMD) has been reported as a key biomechanical property for the assessment of healing in osteoporotic fractures [8,16]. Bone mass, however, is not the only determinant factor for the biomechanical environment, because improvements in the bone microarchitecture, such as the number and spatial arrangement of bone trabeculae, are also important. Bone microarchitecture is also positively affected by HESW therapy for osteoporosis [11]. When tibial fractures occur in rats with osteoporosis, according to the micro-CT results obtained in this study, bone volume (BV), bone volume/tissue volume (BV/TV), average trabecular thickness (Tb.Th), and average number of trabeculae (Tb.N) in the left tibia at 4 and 8 weeks after HESW therapy were about 45.0% and 33.1%, 18.4% and 20.1%, 38.2% and 20.9%, 26.7% and 28.4%, respectively, higher than those in the control group, and the mean trabecular separation (Tb.Sp) was about 16.7% and 27.3%, respectively, lower in the treatment group (Table 2). Besides, callus growth and bone microstructure were also showed superior in the treatment group (Figures 4, 5). In other words, the superior mechanical properties of the treated tibias in osteoporotic rats in the present study reflected increased bone quality after HESW therapy. Therefore, we considered that slightly focused HESWs had a beneficial effect on the restoration and reconstruction of bone microstructure in osteoporotic fractures.
However, the present study has several limitations. First, we set the sham-operated group only to confirm osteoporosis, but not to compare it with OVX + HESW and OVX groups in other test items. Second, because there is no Haversian system in the rat skeleton, there is no way to obtain additional relevant measures such as the porosity of the cortical bone because osteoporosis may be mainly caused by changes in the cortical bone [45]. Third, we did not study the different effects of different energy density and frequency of HESWs on osteoporotic fracture healing. Fourth, we did not compare the effects of HESWs on different sites and types of osteoporotic fractures. Fifth, the study only observed the healing process up to 8 weeks after the HESW treatment, and long follow up may be needed in future studies. Sixth, the study did not investigate the different effects of slightly focused HESWs on osteoporotic fractures of rat tibias in contrast to the focused one.
In conclusion, slightly focused HESW therapy has beneficial effects on osteoporotic fracture healing in rats by promoting callus formation, accelerating the reconstruction of trabecular bone, ameliorating the spatial structure of trabecular bone, and improving the biomechanical properties of healed bone. Besides, it is characterized by wider focal area, larger therapy zone, easier positioning and less pain during treatment, which are more suitable for application in orthopedics. Therefore, slightly focused HESW therapy is a potential adjuvant treatment for osteoporotic fracture. In the future study, clinical experiments involving a large number of subjects are needed to determine the appropriate treatment parameters for humans, which would be more complicated than in rats.
Acknowledgements
Supported by the National Nature Science Foundation of China (Grant No. 81071501) and the Shanghai Committee of Science and Technology, China (Grant No. 09411966500).
Disclosure of conflict of interest
None.
References
- 1.Consensus development conference: prophylaxis and treatment of osteoporosis. Am J Med. 1991;90:107–110. doi: 10.1016/0002-9343(91)90512-v. [DOI] [PubMed] [Google Scholar]
- 2.van Staa TP, Dennison EM, Leufkens HG, Cooper C. Epidemiology of fractures in England and Wales. Bone. 2001;29:517–522. doi: 10.1016/s8756-3282(01)00614-7. [DOI] [PubMed] [Google Scholar]
- 3.Kanis JA, Oden A, Johnell O, Jonsson B, de Laet C, Dawson A. The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int. 2001;12:417–427. doi: 10.1007/s001980170112. [DOI] [PubMed] [Google Scholar]
- 4.Johnell O. The socioeconomic burden of fractures: today and in the 21st century. Am J Med. 1997;103:20S–5S. doi: 10.1016/s0002-9343(97)90023-1. discussion 5S-6S. [DOI] [PubMed] [Google Scholar]
- 5.Jones G, Nguyen T, Sambrook PN, Kelly PJ, Gilbert C, Eisman JA. Symptomatic fracture incidence in elderly men and women: the Dubbo Osteoporosis Epidemiology Study (DOES) Osteoporos Int. 1994;4:277–282. doi: 10.1007/BF01623352. [DOI] [PubMed] [Google Scholar]
- 6.Chrischilles E, Shireman T, Wallace R. Costs and health effects of osteoporotic fractures. Bone. 1994;15:377–386. doi: 10.1016/8756-3282(94)90813-3. [DOI] [PubMed] [Google Scholar]
- 7.Giannoudis P, Tzioupis C, Almalki T, Buckley R. Fracture healing in osteoporotic fractures: is it really different? A basic science perspective. Injury. 2007;38(Suppl 1):S90–99. doi: 10.1016/j.injury.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Namkung-Matthai H, Appleyard R, Jansen J, Hao Lin J, Maastricht S, Swain M, Mason RS, Murrell GA, Diwan AD, Diamond T. Osteoporosis influences the early period of fracture healing in a rat osteoporotic model. Bone. 2001;28:80–86. doi: 10.1016/s8756-3282(00)00414-2. [DOI] [PubMed] [Google Scholar]
- 9.Diamond TH, Clark WA, Kumar SV. Histomorphometric analysis of fracture healing cascade in acute osteoporotic vertebral body fractures. Bone. 2007;40:775–780. doi: 10.1016/j.bone.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Das S, Crockett JC. Osteoporosis-a current view of pharmacological prevention and treatment. Drug Des Devel Ther. 2013;7:435–448. doi: 10.2147/DDDT.S31504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Jagt OP, van der Linden JC, Schaden W, van Schie HT, Piscaer TM, Verhaar JA, Weinans H, Waarsing JH. Unfocused extracorporeal shock wave therapy as potential treatment for osteoporosis. J Orthop Res. 2009;27:1528–1533. doi: 10.1002/jor.20910. [DOI] [PubMed] [Google Scholar]
- 12.Wang CJ. Extracorporeal shockwave therapy in musculoskeletal disorders. J Orthop Surg Res. 2012;7:11. doi: 10.1186/1749-799X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valchanou VD, Michailov P. High energy shock waves in the treatment of delayed and nonunion of fractures. Int Orthop. 1991;15:181–184. doi: 10.1007/BF00192289. [DOI] [PubMed] [Google Scholar]
- 14.Furia JP, Rompe JD, Cacchio A, Maffulli N. Shock wave therapy as a treatment of nonunions, avascular necrosis, and delayed healing of stress fractures. Foot Ankle Clin. 2010;15:651–662. doi: 10.1016/j.fcl.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Rompe JD, Theis C, Maffulli N. Shock wave treatment for tennis elbow. Orthopade. 2005;34:567–570. doi: 10.1007/s00132-005-0805-x. [DOI] [PubMed] [Google Scholar]
- 16.Wang CJ, Yang KD, Wang FS, Hsu CC, Chen HH. Shock wave treatment shows dose-dependent enhancement of bone mass and bone strength after fracture of the femur. Bone. 2004;34:225–230. doi: 10.1016/j.bone.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 17.McClure SR, Van Sickle D, White MR. Effects of extracorporeal shock wave therapy on bone. Vet Surg. 2004;33:40–48. doi: 10.1111/j.1532-950x.2004.04013.x. [DOI] [PubMed] [Google Scholar]
- 18.Mariotto S, Cavalieri E, Amelio E, Ciampa AR, de Prati AC, Marlinghaus E, Russo S, Suzuki H. Extracorporeal shock waves: from lithotripsy to anti-inflammatory action by NO production. Nitric Oxide. 2005;12:89–96. doi: 10.1016/j.niox.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Johannes EJ, Kaulesar Sukul DM, Matura E. High-energy shock waves for the treatment of nonunions: an experiment on dogs. J Surg Res. 1994;57:246–252. doi: 10.1006/jsre.1994.1139. [DOI] [PubMed] [Google Scholar]
- 20.Dorotka R, Kubista B, Schatz KD, Trieb K. Effects of extracorporeal shock waves on human articular chondrocytes and ovine bone marrow stromal cells in vitro. Arch Orthop Trauma Surg. 2003;123:345–348. doi: 10.1007/s00402-003-0551-7. [DOI] [PubMed] [Google Scholar]
- 21.Ma HZ, Zeng BF, Li XL, Chai YM. Temporal and spatial expression of BMP-2 in sub-chondral bone of necrotic femoral heads in rabbits by use of extracorporeal shock waves. Acta Orthop. 2008;79:98–105. doi: 10.1080/17453670710014833. [DOI] [PubMed] [Google Scholar]
- 22.Kearney CJ, Hsu HP, Spector M. The use of extracorporeal shock wave-stimulated periosteal cells for orthotopic bone generation. Tissue Eng Part A. 2012;18:1500–1508. doi: 10.1089/ten.TEA.2011.0573. [DOI] [PubMed] [Google Scholar]
- 23.Forriol F, Solchaga L, Moreno JL, Canadell J. The effect of shockwaves on mature and healing cortical bone. Int Orthop. 1994;18:325–329. doi: 10.1007/BF00180236. [DOI] [PubMed] [Google Scholar]
- 24.Tam KF, Cheung WH, Lee KM, Qin L, Leung KS. Shockwave exerts osteogenic effect on osteoporotic bone in an ovariectomized goat model. Ultrasound Med Biol. 2009;35:1109–1118. doi: 10.1016/j.ultrasmedbio.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Chen YJ, Wurtz T, Wang CJ, Kuo YR, Yang KD, Huang HC, Wang FS. Recruitment of mesenchymal stem cells and expression of TGF-beta 1 and VEGF in the early stage of shock wave-promoted bone regeneration of segmental defect in rats. J Orthop Res. 2004;22:526–534. doi: 10.1016/j.orthres.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Moretti B, Notarnicola A, Garofalo R, Moretti L, Patella S, Marlinghaus E, Patella V. Shock waves in the treatment of stress fractures. Ultrasound Med Biol. 2009;35:1042–1049. doi: 10.1016/j.ultrasmedbio.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Haupt G, Haupt A, Ekkernkamp A, Gerety B, Chvapil M. Influence of shock waves on fracture healing. Urology. 1992;39:529–532. doi: 10.1016/0090-4295(92)90009-l. [DOI] [PubMed] [Google Scholar]
- 28.Alvarez RG, Cincere B, Channappa C, Langerman R, Schulte R, Jaakkola J, Melancon K, Shereff M, Cross GL. Extracorporeal shock wave treatment of non- or delayed union of proximal metatarsal fractures. Foot Ankle Int. 2011;32:746–754. doi: 10.3113/FAI.2011.0746. [DOI] [PubMed] [Google Scholar]
- 29.Altuntas EE, Oztemur Z, Ozer H, Muderris S. Effect of extracorporeal shock waves on subcondylar mandibular fractures. J Craniofac Surg. 2012;23:1645–1648. doi: 10.1097/SCS.0b013e31825e38a2. [DOI] [PubMed] [Google Scholar]
- 30.Petrisor B, Lisson S, Sprague S. Extracorporeal shockwave therapy: A systematic review of its use in fracture management. Indian J Orthop. 2009;43:161–167. doi: 10.4103/0019-5413.50851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang CJ, Huang HY, Chen HH, Pai CH, Yang KD. Effect of shock wave therapy on acute fractures of the tibia: a study in a dog model. Clin Orthop Relat Res. 2001:112–118. doi: 10.1097/00003086-200106000-00015. [DOI] [PubMed] [Google Scholar]
- 32.Foldager CB, Kearney C, Spector M. Clinical application of extracorporeal shock wave therapy in orthopedics: focused versus unfocused shock waves. Ultrasound Med Biol. 2012;38:1673–1780. doi: 10.1016/j.ultrasmedbio.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Kalu DN. The ovariectomized rat model of postmenopausal bone loss. Bone Miner. 1991;15:175–191. doi: 10.1016/0169-6009(91)90124-i. [DOI] [PubMed] [Google Scholar]
- 34.Wronski TJ, Dann LM, Qi H, Yen CF. Skeletal effects of withdrawal of estrogen and diphosphonate treatment in ovariectomized rats. Calcif Tissue Int. 1993;53:210–216. doi: 10.1007/BF01321840. [DOI] [PubMed] [Google Scholar]
- 35.Huang HM, Li XL, Jiang LH, Ding GQ, Liu GJ. Effect of unfocused shock wave on the healing of osteoporotic fractures in rats and the biomechanical evalustion. Chin J Osteoporos. 2012;18:1076–1081. [Google Scholar]
- 36.Huang HM, Li XL, Jiang LH, Liu GJ, Chen XF, Lu CC. Effect of unfocused high energy shock wave on osteoporotic fracture on bone histomorphometry in rats. Chin J Osteoporosis & Bone Miner Res. 2013;6:149–155. [Google Scholar]
- 37.Li XL, Ma HZ, Zeng BF. Effects of high energy shock waves on the bone structre and mechanical strength in experimental osteoporosis. Chin J Osteoporos. 2009;15:317–321. [Google Scholar]
- 38.Chao EY, Inoue N, Koo TK, Kim YH. Biomechanical considerations of fracture treatment and bone quality maintenance in elderly patients and patients with osteoporosis. Clin Orthop Relat Res. 2004:12–25. doi: 10.1097/01.blo.0000132263.14046.0c. [DOI] [PubMed] [Google Scholar]
- 39.Black J, Perdigon P, Brown N, Pollack SR. Stiffness and strength of fracture callus. Relative rates of mechanical maturation as evaluated by a uniaxial tensile test. Clin Orthop Relat Res. 1984:278–288. [PubMed] [Google Scholar]
- 40.Cooper C, Campion G, Melton LJ. Hip fractures in the elderly: a worldwide projection. Osteoporos Int. 1992;2:285–289. doi: 10.1007/BF01623184. [DOI] [PubMed] [Google Scholar]
- 41.Wilson WT, Preminger GM. Extracorporeal shock wave lithotripsy. An update. Urol Clin North Am. 1990;17:231–242. [PubMed] [Google Scholar]
- 42.Rodola F, Conti C, Abballe C, Chierichini A, Ciano F, Forte E, Iacobucci T, Sorrentino L, Vagnoni S, Vergari A, D’Avolis S. Anaesthesia for shock wave therapy in musculoskeletal disorders: a preliminary report. Eur Rev Med Pharmacol Sci. 2002;6:133–138. [PubMed] [Google Scholar]
- 43.Gluer CC, Steiger P, Selvidge R, Elliesen-Kliefoth K, Hayashi C, Genant HK. Comparative assessment of dual-photon absorptiometry and dual-energy radiography. Radiology. 1990;174:223–228. doi: 10.1148/radiology.174.1.2294552. [DOI] [PubMed] [Google Scholar]
- 44.Mazess RB, Barden HS. Measurement of bone by dual-photon absorptiometry (DPA) and dual-energy X-ray absorptiometry (DEXA) Ann Chir Gynaecol. 1988;77:197–203. [PubMed] [Google Scholar]
- 45.Zebaze RM, Ghasem-Zadeh A, Bohte A, Iuliano-Burns S, Mirams M, Price RI, Mackie EJ, Seeman E. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet. 2010;375:1729–1736. doi: 10.1016/S0140-6736(10)60320-0. [DOI] [PubMed] [Google Scholar]


