Abstract
The aim of this study is to investigate the effect of porous tantalum material in repair tibial defects caused by firearm injuries in a rabbit model. A multifunctional biological impact machine was used to establish a rabbit tibial defect model of firearm injury. Porous tantalum rods were processed into a hollow cylinder. Kirschner wires were used for intramedullary fixation. We compared the differences of the bone ingrowth of the porous tantalum material by gross observations, X-rays and histological evaluations. The radiographic observations revealed that fibrous tissue covered the material surface after 4 weeks, and periosteal reactions and new bone callus extending materials appeared after 8 weeks. After 16 weeks, the calluses of the firearm injury group were completely wrapped around a porous tantalum material. The group with the highest Lane-Sandhu X-rays cores was the firearm injury and tantalum implant group, and the blank control group exhibited the lowest scores. The histological evaluations revealed that the presence of new bone around the biomaterial had grown into the porous tantalum. By the 16th week, the areas of bone tissue of the firearm injury group was significant higher than that of non-firearm injury group (P<0.05). The comminuted fractures treated with tantalum cylinders exhibited greater bone ingrowth in the firearm injury group. In conditions of firearm injuries, the porous tantalum biomaterial exhibited bone ingrowth that was beneficial to the treatment of bone defects.
Keywords: Firearm injury, tibial defect, porous tantalum
Introduction
Porous biomaterials have been developed to enhance the biological fixation of bone defects in orthopedic medicine [1-3]. A new type of porous tantalum biomaterial was manufactured by the Zimmer Corporation [4,5]. Clinical results showed that this type of porous tantalum was able to support rapid and extensive bone ingrowth. Porous tantalum has approximately 70% to 80% porous and an average pores size and stiffness which is similar to bone tissue [6]. This material can be made into complex shapes and used as implants in reconstructive orthopedics and other surgical disciplines.
The repair and reconstruction of bone defects caused by firearm injuries are a key topic military medicine. Orthopedic implant is one of the most important applications of porous biomaterials [7-9]. During the treatment of limb fractures in war trauma, the restoration of limb function often plagues orthopedic surgeons [10,11]. Because of the limited bone ingrowth, structural allografts are prone to failure. Bone replacement materials for the repair of these large defects should ideally exhibit bone ingrowth in this complicated biological environment. Because porous tantalum possesses favorable mechanical and biological properties, it has been used in hip and knee artificial joint revisions in which larger bone defects are apparent [12]. However, few studies have explored the efficacy of porous tantalum in the repair of bone defects caused by firearm injuries. The aim of the current study was to characterize the use of this biomaterial for implants for the treatment of bone defects in a small animal model of firearm injuries via radiographic and histological methods.
Materials and methods
Animals
Thirty-six New Zealand white rabbits (either sex, weighing 3 ± 0.2 kg) were provided by the Experimental Animal Center of the Fourth Military Medical University, Xi’an, China. There were no significant differences between the individuals. All experiments were approved by the Ethics Committee of the Fourth Military Medical University and were performed in accordance with the regulations described in the committee’s guiding principles manual. The animals were randomly divided into the following three groups: firearm injury and tantalum implant group (group A), non-firearm injury and tantalum implant group (group B), and firearm injury control group (group C).
Porous tantalum biomaterial
Porous tantalum rods were provided by Zimmer Corporation (Trabecular Metal Technology, Inc. Parsippany, NJ). According to the diameters of the upper and middle tibia, the porous tantalum was mechanically processed into hollow cylindrical biomaterials. The cylinders were prefabricated to match the defect sizes with heights of 10 mm, outer diameters of 8 mm which is the average diameter of rabbits’ tibias and inner diameters of 2.1 mm to allow for the introduction of an intramedullary 2.0 mm Kirschner wire (Figure 1). The actual measured porosity of the biomaterial was approximately 60-75%. All test samples were cleaned in detergent with neutralizers. The samples were then packaged and sterilized by steam.
Figure 1.

Porous tantalum biomaterial specifications. The height was 10 mm, the outer diameter was 8 mm, and a 2.0 Kirschner wire was passed through a central hole with a 2.1 mm diameter hollow area.
Animal model of firearm injury
A firearm injury model was created with a multifunctional bio-impact device. The device was provided by the Department of Oral and Maxillofacial Surgery of the School of Stomatology, Fourth Military Medical University [13,14]. We primarily used this test machine to launch high-speed projectiles. The projectiles were launched into the proximal end of the tube head, and steel ball missile shells were made for projectile loading. We placed a laser speed sensor between the transmitting tubes and the tibias of the rabbits (the distances between the tubes and tibias were 30 cm). The laser pen was used to precisely aim, and the self-excited solenoid valve was opened. The skin of the animal was prepared and weighed before the injury. In the experiment, the animals were anesthetized with 2% sodium pentobarbital (1 ml/kg) via the ear vein and were randomly assigned to receive a left or right leg injury. We hung the injured animals upside down on shelves. Nitrogen pressure was used to control the projectile velocity. The energy of the injury was calculated based on the projectile’s quality and speed [15]. Subsequently, the parameters of the biological impact machine were adjusted (the steel balls were 4 mm in diameter and weighed 0.24 g, the nitrogen pressure was 1.0 MPa, and the projectile velocity was 776.9 ± 19.7 m/s). Finally, the launching of the projectile to hit the target relied on a pressure value controlling compressed nitrogen in the multifunctional bio-impact system. A penetrating wound was made in the animal’s lower leg, and the firearm fractures were of the open comminuted fracture type (Figure 2A). Hemostasis was induced with compression bandages 30 minutes after the injuries.
Figure 2.
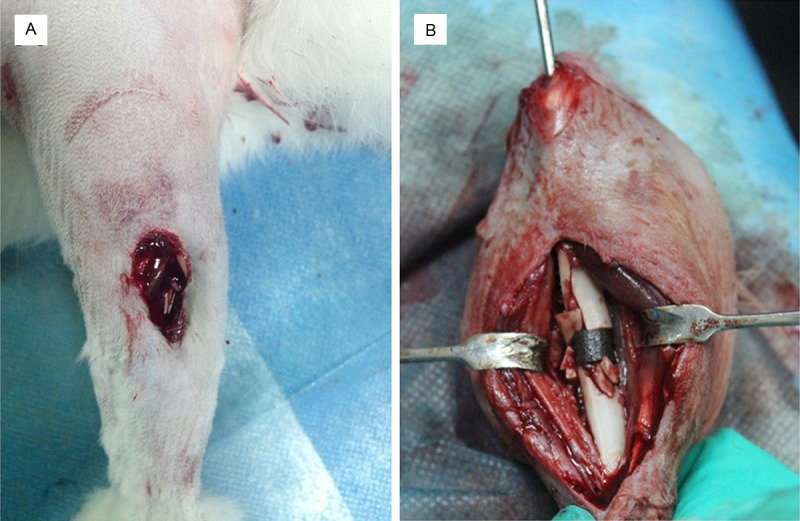
Images of the rabbit tibia firearm wounds (A). Image from a firearm injury group rabbit during porous tantalum implant surgery. Intramedullary Kirschner fixation was used, and fracture chips were placed at the two ends of the biomaterial (B).
Debridement and porous tantalum implant surgery
Based on the principles of the treatment of firearm wounds, debridement was performed 6 hours after the injury [16]. Saline and hydrogen peroxide were used to flush the wound, and the necrotic tissues and muscles were removed. Additionally, a drain was placed in the wound and the wound was not initially closed. The animals were injected intramuscularly with sodium penicillin (400 thousand U/12 h) continuously for one week. Delayed sutures were applied on the third day after injury. Subsequently, the injured limb was placed in a plaster slab for stabilization.
Firearm injury and tantalum implant group: Seven days after the firearm injury, the wound was opened during a second surgery. The obviously necrotic tissue was debrided, and the porous tantalum cylinder was placed to correct the bone defects. In preliminary experiments used to establish the model, the comminuted fractures of the tibias exhibited various shapes, and the average bone defect was 1.02 ± 0.16 cm as measured with a standard ruler. We removed a small amount of bone chips and using a bone-ribbing rongeur, pruned the sharp end of tibial fracture to ensure that the length of the bone defect was about 1 cm. A surgical incision was made in the tubercle of the tibia. We made a hole with an electric drill in the cortical bone of tibia. A 2.0-mm Kirschner wire was pushed into the bone marrow cavity. Next, the Kirschner wire was pushed through the porous tantalum biomaterial and inserted into the lower end of the tibia and a saw blade was used to saw off the tail of the Kirschner wire. The bone defect was replaced by the porous tantalum cylinder, and other bone fragments were placed at the two ends of the biomaterial (Figure 2B).
The non-firearm injury and tantalum implant group: The left or right lower limb was randomly chosen, and a 2-cm skin incision was made based on percutaneous touch positioning of the upper tibia. The subcutaneous tissue was cut layer by layer, and the surrounding blood vessels and nerves were carefully protected. The surgeons bluntly dissected along the muscle space to fully expose the tibia. Subsequently, a 1-cm segment of the defect was measured, and an oscillating saw blade was used for a transverse osteotomy. Saline solution was applied throughout the process to cool the saw and to ensure the complete interception of the bone segments. The resected diaphysis segment of the tibia was removed, and porous tantalum material was used to fill the bone defect. The Kirschner wire fixation was applied in the same manner described for group A. The bleeding in the wound area was stopped, and hydrogen peroxide and sterile saline were used to flush the cut. Next, the wound was closed in steps. A subcutaneous injection of sodium penicillin was administered to avoid infection during the 5 days of postoperative care.
The firearm injury control group also underwent Kirschner intramedullary fixation and firearm wound debridement. No biomaterial was applied to repair the bone defect. Injections of sodium penicillin were administered for seven consecutive days after surgery to prevent infection. In all three groups of animals, the legs were supported with plaster, and the plaster was removed after 4 weeks. The Kirschner wires were removed when the animals were killed.
General observations
The animals were observed after the operation in terms of their diet, activity, local wound healing and other systemic conditions. Four animals from each group were killed at 4, 8 and 16 weeks after wounding. The changes in the biomaterial surfaces and their relationships with the tissue grafts were observed.
X-ray examination
Tibial X-rays were taken at four time points: immediately after the surgery, and in the 4th, 8th and 16th weeks. The extents of the repairs of the bone defects were observed by two experienced orthopedic surgeon and a radiologist, who scored the X-ray films from each group at the different time points independently using the Lane-Sandhu scoring criteria.
Van Gieson (VG) staining
After the removal of the Kirschner fixations, general observations and histological evaluations were sequentially performed on the same specimen. The bone specimens were fixed using 80% ethanol for at least 1 week, decalcified and embedded with plastic. Thereafter, three complete 7-10 μm thick slices were acquired with a using Leica2500 slicer. Each specimen was cut along the longitudinal axis of the porous tantalum. Image acquisitions and assessments of bone callus growth were performed under an optical microscope after Van Gieson (VG) staining. Image Pro Plus 6.0 image software was used to process and analyze the data. The percentages of new bone tissue areas inside the porous tantalum biomaterial were used for statistical analyses (new bone tissue/porous tantalum cylinder area × 100%).
Statistical analyses
Software SPSS 17.0 was used for the statistical analyses. The date are reported as the means ± SEMs. The statistical analyses were performed with a single factor analysis of variance (one-way ANOVA) test. Student-Newman-Keuls method was further used as post hoc test to detect between-group differences. P<0.05 was considered statistically significant.
Results
General observations
Each group of animals gradually recovered in terms of dietary intake, and the wounds healed well. One rabbit with firearm injury died from wound infection. The surfaces of the specimens were covered with fibrous tissue after 4 weeks. A small amount of fibrous callus formation from the bone fragments was observed in group A after 8 weeks. The bone calluses were filled with bone defect areas, and the experimental material junction edge progressively extended to both ends of the material. After 16 weeks, new bone callus had adhered to the biomaterial, and the bone and biomaterial were tightly connected. At both ends of the fracture fragments, the bone calluses had exhibited substantial formation, and new bone had completely wrapped around the porous tantalum cylinder. The ends had formed complete bony connections, and the application of pressure to the two ends revealed no relative movement. The bone callus surrounding the biomaterial in group B was less than that of group A. The rabbits in group C exhibited different degrees of limb shortening deformities; in two cases, fibers wrapping around the fracture and pseudo articulation formation were observed.
X-ray examination
Four weeks after the operations, all of implanted biomaterials were clearly visible in the tibial defect areas. The control group exhibited small areas of high-density patchy shadows in the bone defect areas. No new bone shadows were be observed in either the of the porous tantalum groups at the 4th week after the operations (Figure 3B). In the 8th week after the operations, high-density shadows were observed around the bone fragments at the two ends of the porous tantalum material in group A (Figure 3C). The interface of the fracture fragments and the biomaterial gradually blurred, and the periosteal reaction observed in group A was greater than that observed in group B. However, the Lane-Sandhu scores revealed no significant differences (P>0.05). Over time, the shadows of the periosteal reactions significantly increased and gradually extended to cover the surface of the biomaterial in group A by the 16th week. (Figure 3D). The two ends of the bone fracture had formed a complete connection by synostosis. In contrast, group B exhibited small areas of high-density shadows that were primarily located around the two ends of the biomaterial (Figure 3E).
Figure 3.
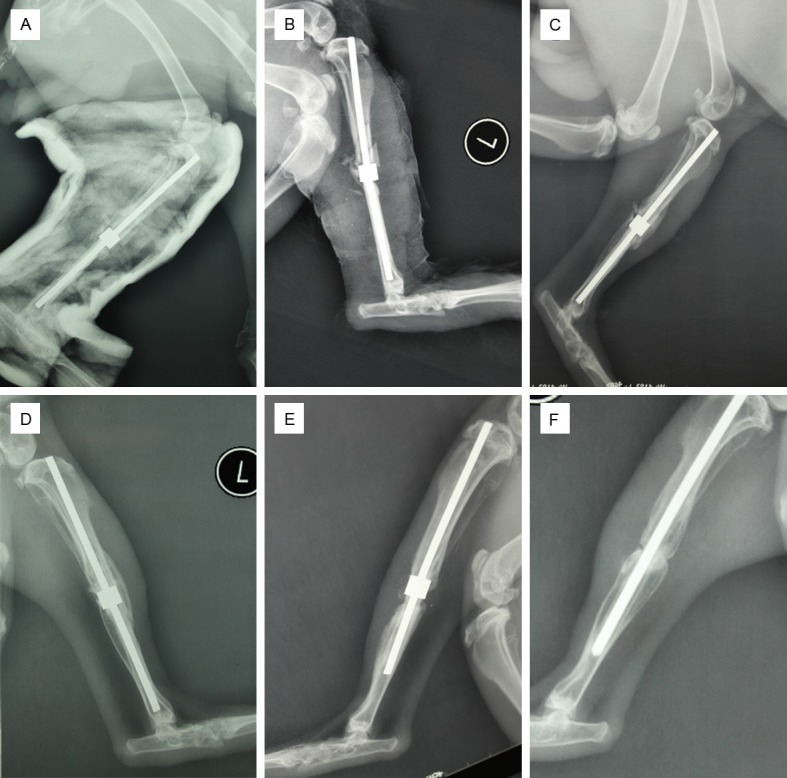
X-ray photographs of the tibia of a rabbit in the firearm injury and implanted tantalum group: after surgery (A); after 4 weeks (B); after 8 weeks (C); after 16 weeks (D). Image from the non-firearm injury and implanted tantalum group at 16 weeks after surgery (E). Image from the firearm injury control group after 16 weeks (F).
The images from group C revealed a series of periosteal reactions during the 8th week. However, after 16 weeks, we found six end scleroses in the areas of the tibial bone defects, a decrease in peripheral bone density and nonunion (Figure 3F). The Lane-Sandhu X-ray scores of the three groups (Figure 4) after 16 weeks revealed that the group A had the highest scores and group C had the lowest. The scores across the three groups were significantly different (P<0.05).
Figure 4.
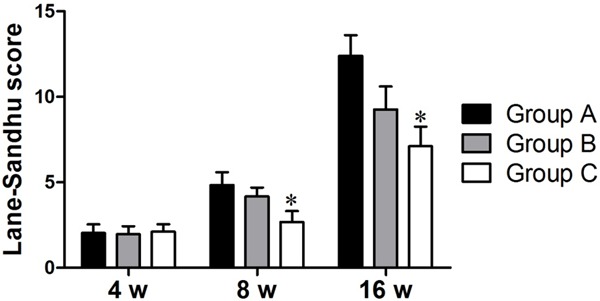
The X-ray bone defect Lane-Sandhu score results for each group after 4 weeks, 8 weeks, and 16 weeks. *P<0.05 vs Group A.
VG staining
In the 4th week after the operations, the bone defect areas of the three groups exhibited extensive fibrous tissue formation. In group A, fibrous septa and inflammatory responses were observed at the interface between the biomaterial and the bone. After 8 weeks, the bone ingrowth had progressed slightly into the edges of porous tantalum cylinders in both groups A and B. The red-dyed new bone and fibrous tissue grew along the pore walls. By the 8th week, the percentages of new bone filling the porous tantalum were not significantly different between groups A and B. At the 16th week, abundant new bone ingrowth into the pores of the biomaterial was observed, and some woven bones were interconnected. In group A, the calluses grew from the periphery into the porous tantalum until the tantalum was completely wrapped (Figure 5A). Group A exhibited more red-dyed new bone within pores than did group B, and this difference was more evident in this period (Figure 5B, 5C).
Figure 5.
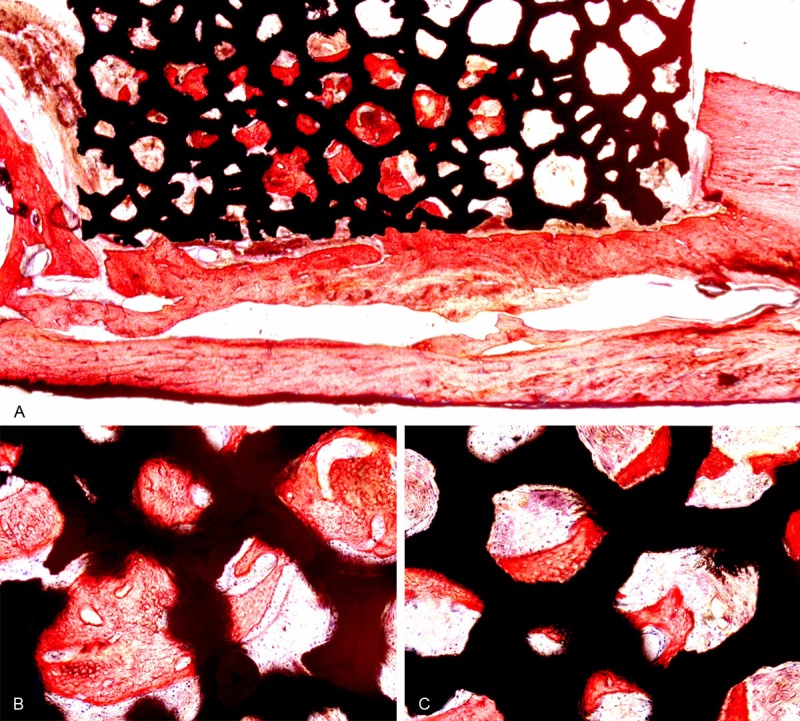
The firearm injury and tantalum implant group in the 16th week, Van Gieson dyed × 16 (A); firearm injury and tantalum implant group: Van Gieson dyed × 100 (B); non-firearm injury and tantalum implant group: Van Gieson dyed × 100 (C).
The morphological analyses of the areas of new bone filling the biomaterial in the 16th week revealed that the firearm injury group exhibited significantly more biomaterial filling than did the non-firearm injury group (P<0.05; Figure 6).
Figure 6.
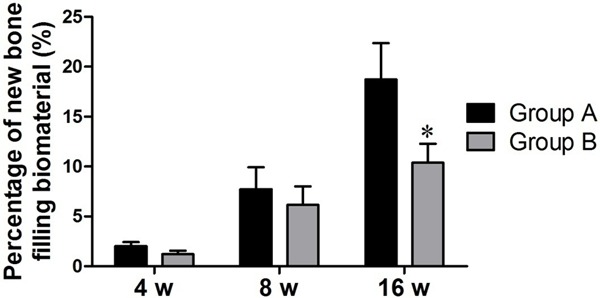
Percentages of new bone filling the biomaterial at 4, 8 and 16 weeks. *P<0.05.
Discussion
In modern military warfare, the penetrating wounds and transient cavity effects caused by high-speed projectiles lead to limb fractures. This is a major issue in military medicine. The traditional methods of treating bone defects include the following: autogenous bone, allograft bone, and tissue-engineered bone transplantation. Because people continually explored the treatment of bone defects, the superiority of various forms of bone graft substitutes gradually stood out. Porous tantalum (trabecular metal) has attracted wide-spread attention due to its relatively high friction coefficient, good ductility, low modulus of elasticity, strong corrosion resistance, excellent biocompatibility and its non-inflammatory interactions with the surrounding tissues following in vivo implantation [4]. An increasing number of applications that exploit the excellent features of porous tantalum have been developed including such as spinal interbody fusion [17], ankle fusion [18,19] and reconstruction of articular cartilage [20]. Therefore, we speculated that porous tantalum could also be applied to reconstruct bone defects caused by firearm injuries.
Because the diameters of conventional bullets are larger than the transverse diameters of the legs of small animals, penetrating wounds inflicted by such bullets in small animal models cannot be effectively treated and the mortality rate is high. We measured the diameters of the upper tibial segments of New Zealand white rabbits (weight 3 ± 0.2 kg) and found the average diameter was approximate 8 mm. Thus, we needed to use small steel balls to create a penetrating wound model. The projection systems of the biological impact machine has been successfully applied in canine maxillofacial firearm injury experiments [14]. Therefore, we used this projection system to establish a rabbit tibial bone defect firearm injury model. In the pre-experiment, we adjusted the projectile velocity and the energy of the injury by manipulating the qualities of the steel ball and the pressure of the nitrogen. By analyzing and comparing the result, we chose the best conditions (i.e., a 4 mm, 0.24 g ball, a nitrogen pressure of 1.0 MPa, and a projectile velocity of 776.9 ± 19.7 m/s).
Stability seems to be an important factor that influences the healing of a firearm wound, particularly when the wound involves an open fracture. In this condition, the reconstruction requires rigid fixation. External fixators have been widely used in the treatment of open fractures caused by firearm injuries, and distraction osteogenesis has been reported in dog experiments [21]. However, the high incidences of pin tract infections, loosening, stiff joints and other complications have also been reported. In contrast to the non-firearm injury experiments of Chou [22], we required strict anti-infection treatments of the firearm injury-induced open wound. We used two phases of wound debridement to remove the necrotic skin and tissue. In the second phase of debridement, we applied intramedullary Kirschner wire fixation. Only one case of wound infection occurred in the firearm injury group, and the skins of the other animals healed within a month.
The outer periosteum is often destroyed in firearm fractures. In contrast to the use of locked plates attached to the bone surface [23,24], intramedullary fixation results in superior reductions of the damage to the periosteum. Bullens [25-27] used porous tantalum intramedullary fixation to repair large bone defects in sheep femurs and achieved good biomechanical performance. Stability is also an important factor that affects fracture healing. In this study, the length of the bone defect was approximately 1 cm, which accounted for approximately 10% of the total length of the rabbit tibia. The Kirschner wire maintained the longitudinal biomechanics, and tubular plaster was used to prevent rotation in the first four weeks. The combined effect of these two methods was satisfactory. However, for small animals, the increase in the length of the bone defect influences the primary stability. This might limit the use of this technique. In the clinic, the percentage of the length of the entire bone is often much higher than this value. This method might not be applicable in such cases, because this kind of reconstruction would likely fail in the long term.
In this study, the X-ray results at the 4th week revealed in conspicuous differences between the groups in terms of the percentages of biomaterial exhibiting bone ingrowth. After 8 weeks, the radiological results revealed abundant external callus around the tantalum cylinder implant in the firearm injury group. This secondary bone healing caused by the relative instability of the reconstruction with a Kirschner internal fixation induced more callus formation. This phenomenon is well known in fracture healing studies. The percentage of bone significantly increased in the firearm injury group as indicated by the histological analyses.
Bone defect repair primarily depends on the host bone cells invading the material gaps. This invasion was sustainably increased in terms of the bone ingrowth observed in the firearm injury group at the 16th weeks, but the results showed no significant increase in the non-firearm injury group. The percentage of bone tissue areas inside the biomaterials in the firearm injury group was significant higher than that of non-firearm injury group. This phenomenon can be explained as follows. The bone chips of the comminuted fractures in the firearm injuries that were implanted in both ends of the material increased the contact area between the host bone and porous tantalum. Although the porous tantalum biomaterial was blocked at the edge and exhibited reduced porosity, in the organizational environment of the firearm wounds which involved muscle tears, this alteration in the porosity of the biomaterial did not affect the bone ingrowth process.
In tantalum cylinder reconstruction, the bone probably does not need to reach the center of the defect to achieve adequate stability. The fractures induced by firearm injuries led to the formation of more external callus as shown in our histological analyses. However, the formation of bone defects in the non-firearm injury group relied on osteotomy. The host bone was completely dependent on clean bone fracture ends for the porous tantalum material to grow. Thus, among these two conditions, the firearm injury group exhibited faster bone ingrowth, but this result requires further evaluation involving long-term observations.
Conclusion
The use of biomaterials to repair bone defects is a long and complicated process. This process depends on porosity and the ability to induce bone ingrowth. This process is related to the body’s own regulation and expression of osteogenic factors. This study was based on a firearm injury model in small animals and the use of intramedullary Kirschner fixation which provided stability to the porous tantalum biomaterials. Porous tantalum bone ingrowth characteristics that are favorable for the treatment of bone defects induced by firearm injuries. Moreover, this experiment provided a theoretical basis for the application of porous tantalum biomaterial to the repair of large bone defects.
Acknowledgements
The authors acknowledge Fuhang Li and Gang Hu for their technical expertise in preparing the histological sections.
Disclosure of conflict of interest
None.
References
- 1.Yavari SA, Wauthle R, van der Stok J, Riemslag AC, Janssen M, Mulier M, Kruth JP, Schrooten J, Weinans H, Zadpoor AA. Fatigue behavior of porous biomaterials manufactured using selective laser melting. Mater Sci Eng C Mater Biol Appl. 2013;33:4849–4858. doi: 10.1016/j.msec.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Jansen EJ, Sladek RE, Bahar H, Yaffe A, Gijbels MJ, Kuijer R, Bulstra SK, Guldemond NA, Binderman I, Koole LH. Hydrophobicity as a design criterion for polymer scaffolds in bone tissue engineering. Biomaterials. 2005;26:4423–4431. doi: 10.1016/j.biomaterials.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Ramrattan NN, Heijkants RG, van Tienen TG, Schouten AJ, Veth RP, Buma P. Assessment of tissue ingrowth rates in polyurethane scaffolds for tissue engineering. Tissue Eng. 2005;11:1212–1223. doi: 10.1089/ten.2005.11.1212. [DOI] [PubMed] [Google Scholar]
- 4.Bobyn JD, Stackpool GJ, Hacking SA, Tanzer M, Krygier JJ. Characteristics of bone ingrowth and interface mechanics of a new porous tantalum biomaterial. J Bone Joint Surg Br. 1999;81:907–914. doi: 10.1302/0301-620x.81b5.9283. [DOI] [PubMed] [Google Scholar]
- 5.Balla VK, Bodhak S, Bose S, Bandyopadhyay A. Porous tantalum structures for bone implants: fabrication, mechanical and in vitro biological properties. Acta Biomater. 2010;6:3349–3359. doi: 10.1016/j.actbio.2010.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine BR, Sporer S, Poggie RA, Della Valle CJ, Jacobs JJ. Experimental and clinical performance of porous tantalum in orthopedic surgery. Biomaterials. 2006;27:4671–4681. doi: 10.1016/j.biomaterials.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 7.Kujala S, Ryhanen J, Danilov A, Tuukkanen J. Effect of porosity on the osteointegration and bone ingrowth of a weight-bearing nickel-titanium bone graft substitute. Biomaterials. 2003;24:4691–4697. doi: 10.1016/s0142-9612(03)00359-4. [DOI] [PubMed] [Google Scholar]
- 8.Van der Stok J, Van der Jagt OP, Amin Yavari S, De Haas MF, Waarsing JH, Jahr H, Van Lieshout EM, Patka P, Verhaar JA, Zadpoor AA, Weinans H. Selective laser melting-produced porous titanium scaffolds regenerate bone in critical size cortical bone defects. J Orthop Res. 2013;31:792–799. doi: 10.1002/jor.22293. [DOI] [PubMed] [Google Scholar]
- 9.van der Stok J, Wang H, Amin Yavari S, Siebelt M, Sandker M, Waarsing JH, Verhaar JA, Jahr H, Zadpoor AA, Leeuwenburgh SC, Weinans H. Enhanced bone regeneration of cortical segmental bone defects using porous titanium scaffolds incorporated with colloidal gelatin gels for time- and dose-controlled delivery of dual growth factors. Tissue Eng Part A. 2013;19:2605–2614. doi: 10.1089/ten.TEA.2013.0181. [DOI] [PubMed] [Google Scholar]
- 10.Frank M, Mathieu L. [Management of war orthopaedic injuries in recent armed conflicts] . Acta Chir Orthop Traumatol Cech. 2013;80:197–202. [PubMed] [Google Scholar]
- 11.Mattila VM, Makitie I, Pihlajamaki H. Trends in hospitalization for firearm-related injury in Finland from 1990 to 2003. J Trauma. 2006;61:1222–1227. doi: 10.1097/01.ta.0000197179.50226.1d. discussion 1227. [DOI] [PubMed] [Google Scholar]
- 12.Patil N, Lee K, Goodman SB. Porous tantalum in hip and knee reconstructive surgery. J Biomed Mater Res B Appl Biomater. 2009;89:242–251. doi: 10.1002/jbm.b.31198. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Lei D, Liu G, He L, Zhou S. Development of bio-multi-function impact device. Journal of Practical Stomatology. 2003;19:269–270. [Google Scholar]
- 14.Liu YQ, Chen XY, Li SG, Chen XM, Guo RF, Wang DT, Fu XB, Jiang SP, Xu GW. Wounding effects of small fragments of different shapes at different velocities on soft tissues of dogs. J Trauma. 1988;28:S95–98. doi: 10.1097/00005373-198801001-00021. [DOI] [PubMed] [Google Scholar]
- 15.Maiden N. Ballistics reviews: mechanisms of bullet wound trauma. Forensic Sci Med Pathol. 2009;5:204–209. doi: 10.1007/s12024-009-9096-6. [DOI] [PubMed] [Google Scholar]
- 16.Tosti R, Rehman S. Surgical management principles of gunshot-related fractures. Orthop Clin North Am. 2013;44:529–540. doi: 10.1016/j.ocl.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Zou X, Li H, Bunger M, Egund N, Lind M, Bunger C. Bone ingrowth characteristics of porous tantalum and carbon fiber interbody devices: an experimental study in pigs. Spine J. 2004;4:99–105. doi: 10.1016/s1529-9430(03)00407-8. [DOI] [PubMed] [Google Scholar]
- 18.Adams JE, Zobitz ME, Reach JS Jr, An KN, Lewallen DG, Steinmann SP. Canine carpal joint fusion: a model for four-corner arthrodesis using a porous tantalum implant. J Hand Surg Am. 2005;30:1128–1135. doi: 10.1016/j.jhsa.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Frigg A, Dougall H, Boyd S, Nigg B. Can porous tantalum be used to achieve ankle and subtalar arthrodesis?: a pilot study. Clin Orthop Relat Res. 2010;468:209–216. doi: 10.1007/s11999-009-0948-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mrosek EH, Schagemann JC, Chung HW, Fitzsimmons JS, Yaszemski MJ, Mardones RM, O’Driscoll SW, Reinholz GG. Porous tantalum and poly-epsilon-caprolactone biocomposites for osteochondral defect repair: preliminary studies in rabbits. J Orthop Res. 2010;28:141–148. doi: 10.1002/jor.20983. [DOI] [PubMed] [Google Scholar]
- 21.Tian L, He L, Liu R, Ren C. Experiment study of distraction osteogenesis for rehabilitation of mandible defect injured by high velocity projectile in dogs. Journal of Traumatic Surgery. 2009;11:438–442. [Google Scholar]
- 22.Chou TG, Petti CA, Szakacs J, Bloebaum RD. Evaluating antimicrobials and implant materials for infection prevention around transcutaneous osseointegrated implants in a rabbit model. J Biomed Mater Res A. 2010;92:942–952. doi: 10.1002/jbm.a.32413. [DOI] [PubMed] [Google Scholar]
- 23.Li BC, Zhang JJ, Xu C, Zhang LC, Kang JY, Zhao H. Treatment of rabbit femoral defect by firearm with BMP-4 gene combined with TGF-beta1. J Trauma. 2009;66:450–456. doi: 10.1097/TA.0b013e3181848cd6. [DOI] [PubMed] [Google Scholar]
- 24.Poultsides LA, Papatheodorou LK, Karachalios TS, Khaldi L, Maniatis A, Petinaki E, Malizos KN. Novel model for studying hematogenous infection in an experimental setting of implant-related infection by a community-acquired methicillin-resistant S. aureus strain. J Orthop Res. 2008;26:1355–1362. doi: 10.1002/jor.20608. [DOI] [PubMed] [Google Scholar]
- 25.Bullens PH, Bart Schreuder HW, de Waal Malefijt MC, Verdonschot N, Buma P. Is an impacted morselized graft in a cage an alternative for reconstructing segmental diaphyseal defects? Clin Orthop Relat Res. 2009;467:783–791. doi: 10.1007/s11999-008-0686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bullens PH, Hannink G, Verdonschot N, Buma P. No effect of dynamic loading on bone graft healing in femoral segmental defect reconstructions in the goat. Injury. 2010;41:1284–1291. doi: 10.1016/j.injury.2010.07.247. [DOI] [PubMed] [Google Scholar]
- 27.Bullens PH, Schreuder HW, de Waal Malefijt MC, Verdonschot N, Buma P. The presence of periosteum is essential for the healing of large diaphyseal segmental bone defects reconstructed with trabecular metal: a study in the femur of goats. J Biomed Mater Res B Appl Biomater. 2010;92:24–31. doi: 10.1002/jbm.b.31485. [DOI] [PubMed] [Google Scholar]


