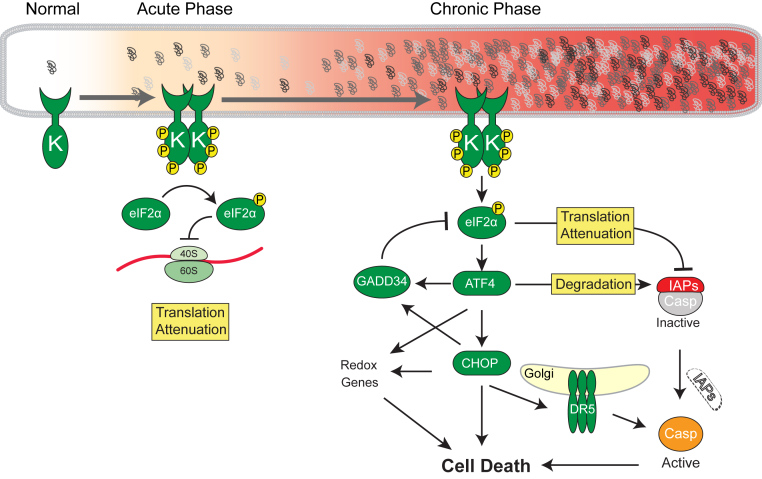
Figure 3.
Consequences of acute and chronic PKR-like endoplasmic reticulum (ER) kinase (PERK) activation. PERK has a kinase domain (K), and phosphorylates eukaryotic translation initiation factor 2 subunit alpha (eIF2α). In the acute phase, PERK-eIF2αP attenuates overloading the proteins into ER. On chronic activation of PERK signaling, expression of activating transcription factor-4 (ATF4) is transnationally up-regulated, which regulates cell fate. GADD34 dephosphorylates eIF2αP to eIF2α, and protein translation is reinitiated. Expression of ATF4 causes oxidative stress. Proapoptotic transcription factor CHOP is transcriptionally induced by ATF4, and its translation is also enhanced by ATF4. Death receptor 5 (DR5; official name TNFRSF10B) is a CHOP target gene, and abundant DR5 protein forms oligomer at the Golgi apparatus, which activates caspase (Casp)8 without requirement of any ligand. Inhibitors of apoptosis proteins (IAPs) are key cell death regulators in metazoans, through their suppression of caspases. Recent studies link PERK-eIF2αP-ATF4 signaling to IAP regulation during ER stress. In response to chronic ER stress, IAP levels decrease specifically through the actions of PERK, but not IRE1 or ATF6 branches of the unfolded protein response. The eIF2αP attenuates de novo IAP synthesis, particularly X-linked IAP, and ATF4's transcriptional activity destabilizes extant XIAP protein.