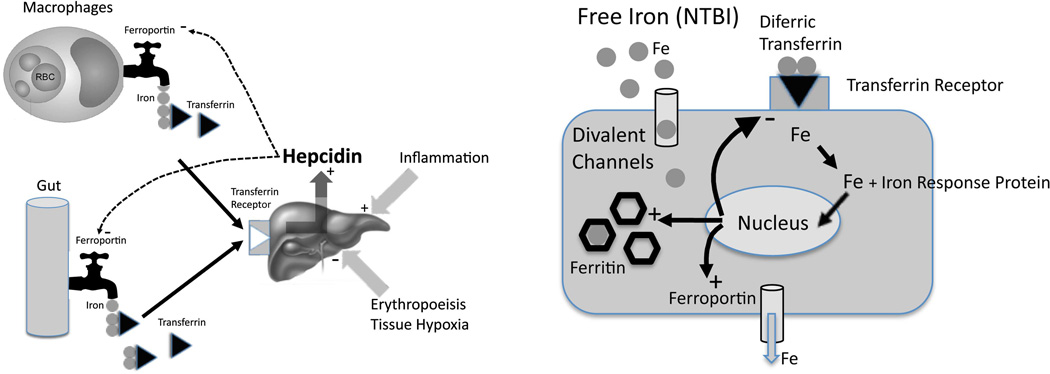
Figure 1.
(Left) Schematic illustrating regulation of total body iron. Elevated iron flux through the gut and reticuloendothelial system increases transferrin saturation, upregulating hepcidin production in the liver. Hepcidin, by triggering internalization of ferroportin, limits iron export into the blood, serving as the central counter-regulatory mechanism. Erythropoietic drive suppresses hepcidin, thereby increasing iron flux from the gut and reticuloendothelial system. Inflammatory cytokines have the opposite effect. (Right) Schematic illustrating iron balance at the cellular level. When transferrin is not fully saturated, all iron entering parenchymal cells occurs through the transferrin receptor. Iron response proteins “sense” intracellular iron, and modulate gene expression of transferrin receptor, ferritin, and ferroportin to properly balance import, storage and export of labile iron. When transferrin is fully saturated, circulating non-transferrin-bound iron (NTBI) can pass unrestricted through divalent cation channels, overwhelming the cells ability to store and export iron.