Figure 2. Sp1, Sp3 and NFκB bind to the endogenous c-FLIP promoter.
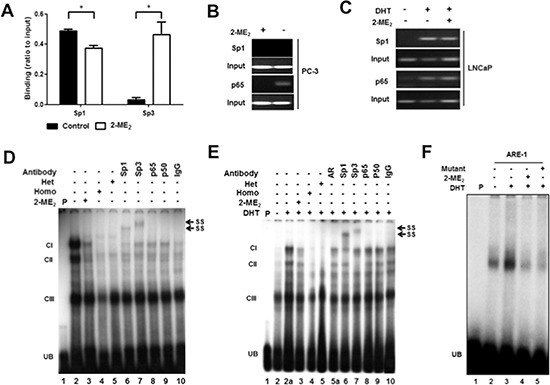
A. DU145 cells were untreated or treated with 2-ME2 (5 μM) for 24 h, and chromatin immunoprecipitation quantitative real-time PCR (ChIP-qPCR) was performed using anti-Sp1 or anti-Sp3 antibody. The amplification value from immunoprecipitated DNA was normalized to 10% input. Error bars indicate ± S.E.M. (n = 3). *p < 0.05. B. PC-3 cells were untreated or treated with 2-ME2 (5 μM) for 24 h. C. LNCaP cells were untreated or treated with 2-ME2 (3 μM) for 6 h in the presence or absence of DHT (1 nM). Gel-based ChIP-PCR was performed using anti-Sp1 or anti-p65 antibody. D. Binding of AR, Sp1, Sp3 and p65 to the c-FLIP promoter sequence elements containing Sp1, NFκB, or AR binding sites to nuclear extracts prepared from PC-3 cells using electrophoretic mobility shift assay (EMSA). PC-3 cells untreated or treated with 2-ME2 (3 μM) for 24 h. Radiolabeled c-FLIP probe was preincubated with 100-fold molar excess of unlabeled c-FLIP sequence (homologous) or Sp1 oligonucleotide with mutation (heterologous) for 5 min prior to incubation with nuclear extracts. Nuclear extracts pre-incubated with indicated antibodies for 30 minutes on ice were used in super-shift experiments. E. Nuclear extracts prepared from LNCaP cells untreated or pretreated with 2-ME2 (3 μM) for 6 h prior to stimulation with DHT (1 nM) for 1 h were used in EMSA. EMSA was carried out essentially as described above. F. Binding of nuclear extracts from untreated or 5α-DHT stimulated LNCaP cells to c-FLIP ARE-1 (+57/+71) as radiolabeled probe was shown. (CI-III: DNA-protein complexes, UB: unbound free probe, SS: super-shifted bands).
