Figure 1.
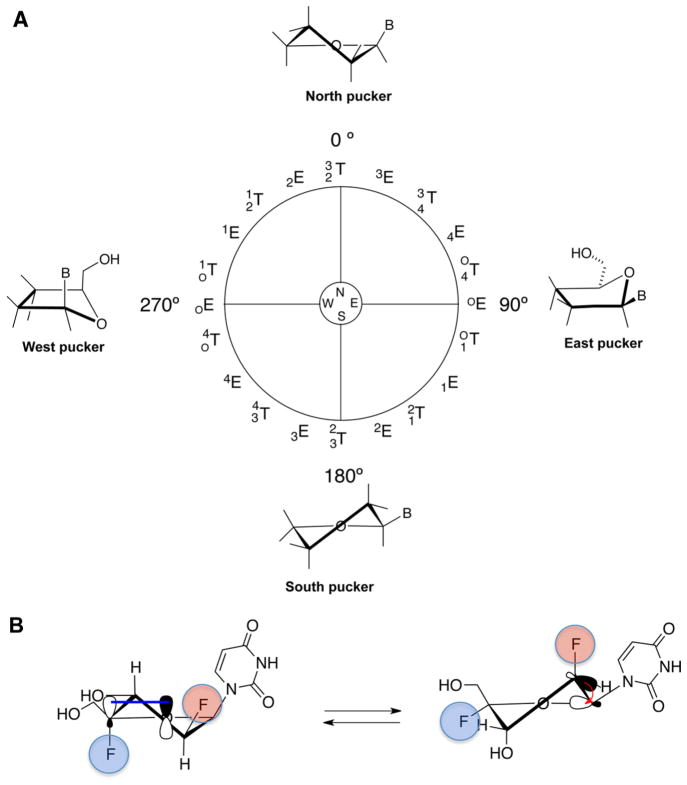
(A) Pseudorotational cycle describing the sugar conformations of nucleosides (E, envelope; T, twist). Superscripts and subscripts indicate the specific atoms in the ribose ring that project away from the plane defined by the remaining ring atoms. Natural nucleosides have characteristic minima in the North (0–36°) and South (144–180°) regions. (B) Anomeric effect (left) favoring the North conformation due to the overlap of a lone-pair orbital O4′(p-type) with the σ*C4′–F4′ antibonding orbital and gauche effect (right) favoring the South conformation due to the interaction between σ*C2′–H2′ bonding orbital and the σ*C1′–O4′ antibonding orbital.