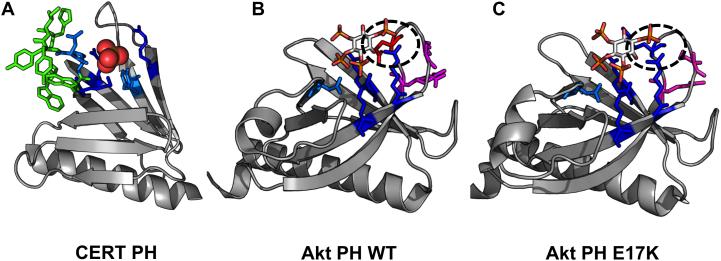
Figure 4. Pleckstrin Homology (PH) domains have a β-barrel structure with an alpha helical cap.
These structures have been crystallized with either sulfate of inositol headgroups and demonstrate PI-binding at one end of the barrel with positively-charged residues and hydrophobic residues which insert into the membrane from the loop regions of the protein. PI-binding residues are depicted in blue. Dark blue residues are positively charged lysines or argininges and light blue are other inositol-headgroup coordinating residues. Residues highlighted in green insert themselves into the membrane from the loop regions, and residues highlighted in magenta are PS-binding. A. CERT PH (4HHV) coordinates a sulfate ion (orange and yellow spheres) in the inositol-binding region with Lys32, Thr34, Asn35, Arg43, Tyr54, Arg66 and inserts into the membrane with Trp33, Tyr36, Ile37, His38, Trp40. B. Akt PH (1UNQ) coordinates IP4 with Glu17, Arg23, Arg25, Asn52, and Arg86. It coordinates PS with Arg15 and Lys20. C. Akt PH (2UZS) is known to be converted to a constitutively active form in some cancers via mutation of Glu17 to Lys17 (highlighted by the black ovals in B and C).