Figure 4. Mechanism of SML action.
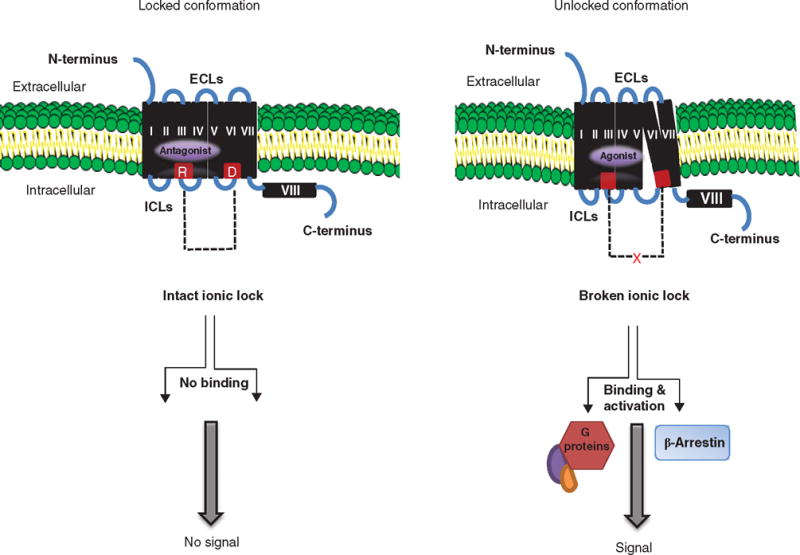
The basal state of TSHR activity is hypothesized to be constrained by polar interactions between different TMHs forming an ‘ionic lock’ between an arginine (R) at the base of transmembrane TMH3 and partly conserved residues such as glutamate (E) or aspartic acid (D) at the base of TM6 as shown in red (left panel). When an SML antagonist binds to the allosteric pocket (purple) on the TMD, the ionic lock remains intact even if TSH ligand binds to the ectodomain because there can be no outward movement of TMH6. Hence, the basal conformation of the receptor is locked and stabilized. In contrast, when a SML agonist binds to the allosteric pocket (right panel), there is an outward movement of TMH6 as a result of destabilization of the ‘ionic lock’ and loss of constrained polar interactions. This movement causes conformational changes in the ICL, which, in turn, allow binding of G proteins and β-arrestin activation leading to signal transduction.
Reproduced with permission from [72].
ECL: Extracellular loops; ICL: Intracellular loops; SML: Small molecule ligands; TMD: Transmembrane domain; TMH: Transmembrane helice; TSHR: Thyroid-stimulating hormone receptor.
