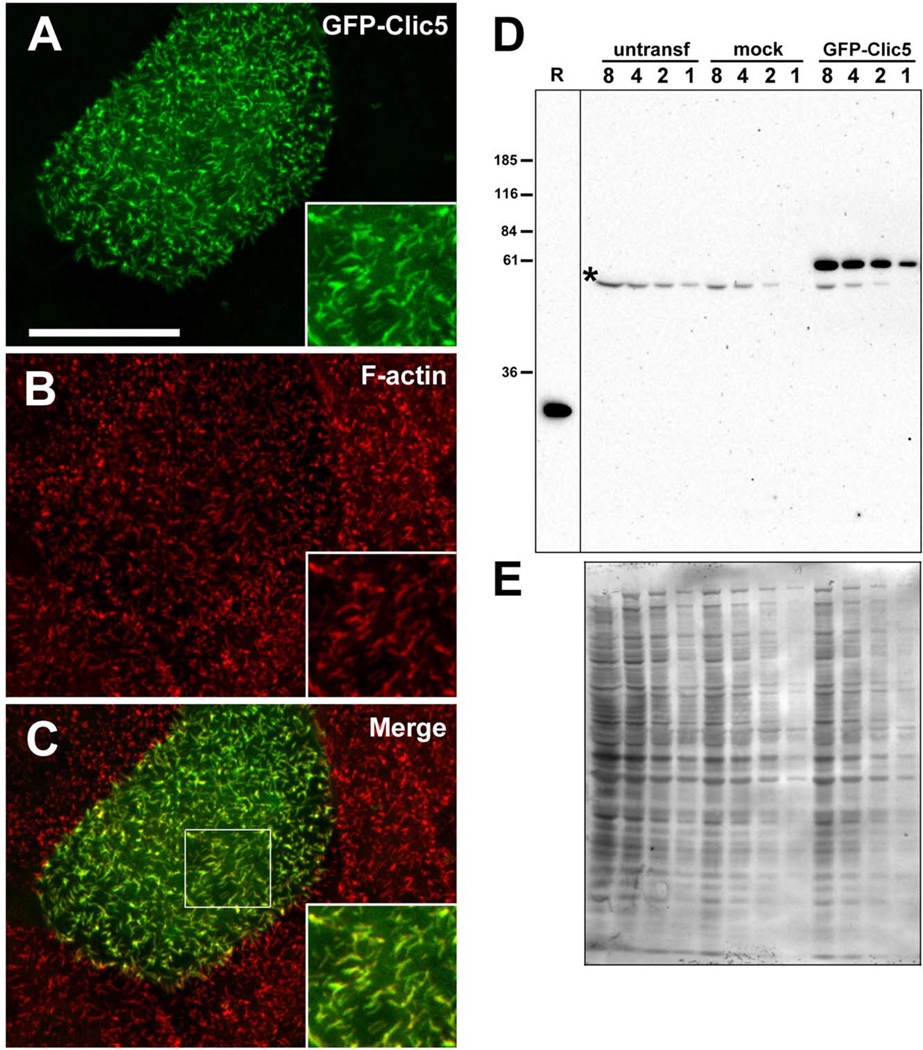
Fig. 4.
Characterization of GFP-CLIC5 fusion protein. (A–C) LLC-PK1-CL4 epithelial cell transfected with GFP-CLIC5 (A) and counterstained with phalloidin (B). Merged images (C) with boxed area to indicate region shown in insets. GFP-CLIC5 concentrates in microvilli-like surface structures rich in F-actin. Scale bar: 20 µm. (D) Western blot of LLC-PK1-CL4 extracts probed with whole antiserum (B132) against CLIC5. Blot contains two-fold serial dilutions (lanes 8, 4, 2, 1) of extracts from untransfected (untransf), mock-transfected cells treated with transfection reagent with no DNA and GFP-CLIC5 transfected cultures. Bacterial lysate containing 50 ng untagged human CLIC5 (R) was loaded on the same gel as a positive control. A band migrating between ~55 and 60 kDa and corresponding to the expected size of the fusion protein is detected only in the GFP-CLIC5 culture; a faster migrating background band (*) is seen in all cultures. Note that no endogenous CLIC5 (~32 kDa) was detected. (E) Western blot stained with Coomassie Blue as a loading control. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]