Abstract
Diabetic nephropathy (DN) often develops in patients suffering from type 1 or type 2 diabetes mellitus. DN is characterized by renal injury resulting in proteinuria. Neuropilin-1 (NRP-1) is a single-pass transmembrane receptor protein devoid of enzymatic activity. Its large extracellular tail is structured in several domains, thereby allowing the molecule to interact with multiple ligands linking NRP-1 to different pathways through its signaling co-receptors. NRP-1’s role in nervous system development, immunity, and more recently in cancer, has been extensively investigated. Although its relation to regulation of apoptosis and cytoskeleton organization of glomerular vascular endothelial cells was reported, its function in diabetes mellitus and the development of DN is less clear. Several lines of evidence demonstrate a reduced NRP-1 expression in glycated-BSA cultured differentiated podocytes as well as in glomeruli from db/db mice (a model of type 2 Diabetes) and in diabetic patients diagnosed with DN. In vitro studies of podocytes implicated NRP-1 in the regulation of podocytes’ adhesion to extracellular matrix proteins, cytoskeleton reorganization, and apoptosis via not completely understood mechanisms. However, the exact role of NRP-1 during the onset of DN is not yet understood. This review intends to shed more light on NRP-1 and to present a link between NRP-1 and its signaling complexes in the development of DN.
Keywords: NRP-1, diabetic nephropathy, AGEs, VEGF-A, Sema3A
1. Introduction
Diabetic nephropathy (DN) is a well-known complication that occurs in diabetic patients with type 1 and type 2 diabetes mellitus in the course of disease [1,2,3]. DN is characterized by increased proteinuria and subsequently declining renal function [1,3]. Prolonged and uncontrolled hyperglycemia contributes to an accumulation of advanced glycation end-products [4,5,6], elevated angiotensin II (ANG II) levels, hypertension [7,8], chronic inflammation, and augmented generation of profibrotic cytokines such as transforming growth factor—beta1 (TGF-β1) [9]—as well as vascular endothelial growth factor-A (VEGF-A), all of which contribute to the development of DN [10]. Some of the most reported pathological changes in DN are a thickening of the glomerular basement membrane (GBM), glomerular hypertrophy, glomerulosclerosis, podocytes foot process effacement and increased podocytes loss, mesangial cells expansion, and tubulointerstitial fibrosis [1,2,11].
These pathophysiological changes result from altered gene and protein expressions of numerous targets or modulation of the physiological signaling cascades, thus leading to the onset and progression of DN. The neuropilins (NRPs), neuropilin-1 (NRP-1) and its homologue neuropilin-2 (NRP-2), are receptor molecules that bind various ligands via a large extracellular part that consists of several domains [12]. Although originally detected in neurons [13,14], neuropilins are also expressed in non-neuronal cells including renal cells [15,16,17,18]. Due to the absence of catalytic activity NRPs need co-receptors to transduce active signals into the cells, therefore they form complexes with a number of signaling co-receptors [12]. Albeit some of NRPs’ ligands and particularly those of NRP-1 are implicated in diabetes and DN, the role of NRP-1 is not yet completely elucidated. The aim of this review is to shed more light on the function of NRP-1 in culture podocytes and to present a link between NRP-1 and its signaling complexes in the development and progression of DN.
2. Neuropilin-1 Structure
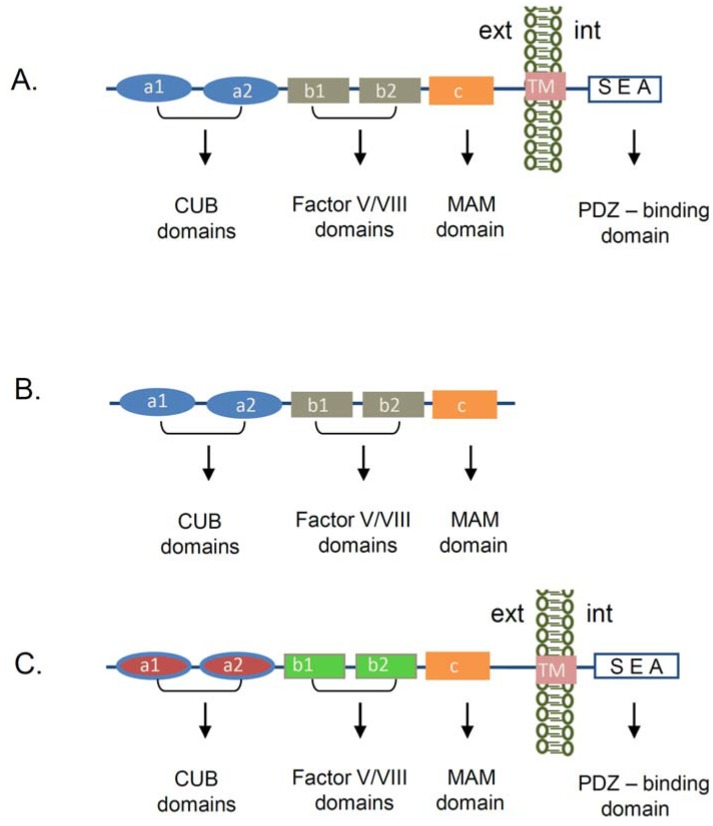
Neuropilin-1 (NRP-1) was first discovered as an antigen that binds to the A5-antibody, raised against neuronal cell surface proteins in the nervous system [19]. It was originally reported that NRP-1 functions as an adhesion receptor in the nervous system [13]. Subsequently, studies revealed that NRP-1 is implicated into the axon guidance through its association with semaphorin III (Sema III) family of proteins [20,21,22]. In addition to the nervous system the expression of NRP-1 was also reported in the heart [23], endothelial cells [24], tumor cells [25], stromal cells [26], and T cells [27,28]. We and others also detected NRP-1 mRNA and protein expression in renal cells [15,16,17,18]. NRP-1 is a transmembrane protein, consisting of a large extracellular part and a short intracellular tail. While its domain structure shares about 44% amino acid homology and structural similarity with the neuropilin-2 (NRP-2) protein, the molecules differ in regard to their function and ligand binding [14] (Figure 1). The NRP-1 extracellular region is organized in five domains: two CUB domains, two Factor V/VIII domains, and a MAM domain, followed by a transmembrane domain and an intracellular tail (Figure 1A). The CUB domains (a1/a2) share homology with complement binding factors C1s/C1r [29], Uegf (urchin embryonic growth factor) [30], and the bone morphogenic protein 1 (BMP1) [31]. In the complement system the CUB domains are known to induce protein-protein interactions, which are mainly regulated by the formation of the immunoglobulin-like structures. Several CUB-domain containing proteins were implicated in the regulation of the cellular adhesion and motility [32,33]. Structural studies have shown that a1/a2 domains of NRP-1 participate in the binding to Semaphorin ligand(s) [34], while the two Factor V/VIII (b1/b2) domains promote the association with another NRP-1 ligand VEGF-A164 in mouse or VEGF-A165 in humans [35]. The NRP-1 extracellular domain crystal structure revealed that the b1 domain is necessary for the VEGF-A164 binding, whereas the b2 domain is mainly involved in the stabilization and coordination of the binding between NRP-1 and its ligands Sema3A or VEGF-A164 [34]. The so named MAM (c) domain, displays homologies with the extracellular regions of mephrin [36], A5 antigen, and the receptor tyrosine phosphatase—µ [37]. This part of the protein is hypothesized to play an important role in the NRP-1 homodimerization, due to its capacity to induce homophilic interactions [38]. Structural studies of the transmembrane part of the NRP-1 depicted a putative GxxxG motif, which is thought to participate in receptor dimerization or oligomerization [39]. The intracellular SEA amino acid sequence in NRP-1,2 is a consensus region shown do promote association with the PSD-95/Dlg/ZO-1 (PDZ) domain containing proteins as the neuropilin interacting protein-1 (NIP1) termed also synectin or RGS-GAIP-interacting protein (GIPC) [40].
Figure 1.
Schematic presentation of neuropilin’s structure. (A) Domain organization of the transmembrane Neuropilin-1 (NRP-1) receptor. The extracellular part of the NRP-1 receptor is organized in several domains. The a1/a2 domains (CUB domains) are required for the binding to semaphorin 3A (SEMA3A) ligand, while the b1/b2 domains (Factor V/VIII domains) are involved in the association with the Vascular Endothelial Growth Factor-A (VEGF-A). The c domain (MAM domain) plays an important role in the NRP-1 homophilic interaction and oligomerization. The transmembrane domain (TM) is important for dimerization and oligomerization of the protein. The last amino acids of the cytoplasmic part (SEA) confer a consensus sequence, which interacts with a PDZ-domain containing proteins. (B) Soluble NRP-1 receptor structure. The molecule consists of the same extracellular structure as the transmembrane NRP-1 receptor, but is missing the TM and the cytoplasmic part of the NRP-1 receptor. (C) NRP-2 transmembrane protein—domain organization. Both NRP-1 and NRP-2 share 44% amino acid homology. NRP-2 consists of the same domains as NRP-1 but binds different signaling ligands and co-receptors. (CUB—C1s/C1r, Uegf (urchin embryonic growth factor), and the Bone morphogenic protein 1 (BMP1), MAM—mephrin, A5 antigen, receptor tyrosine phosphatase—μ, PDZ—PDS-95/Dlg/ZO-1 domain, TM—transmembrane, ext—extracellular environment, int—intracellular environment).
In addition, NRP-1 is also presented in a soluble form, missing the cytoplasmic tail and the transmembrane region of the molecule (Figure 1B). Its role is not well characterized, but it is suggested that it can function as a decoy receptor for NRP-1 ligands [41].
3. Neuropilin-1 Ligands and Signaling Co-Receptors
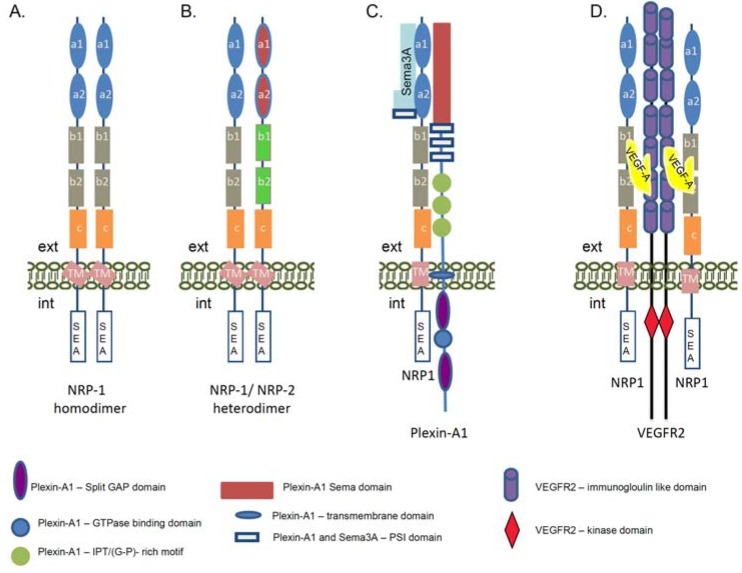
The well-structured extracellular part of the NRP-1 receptor suggests its involvement in numerous extracellular interactions and signaling pathways. NRP-1 has no catalytic activity, therefore to transduce signals into the cells it must associate with multiple ligands and signaling co-receptors (Figure 2A–D). In neurons, NRP-1 is essential for distribution of the down-stream signals initiated from class III semaphorin (Sema3) family of axon guidance molecules [20,21,41,42]. NRP-1 specifically binds to Sema3A [43], while its homolog NRP-2 interacts with Sema3F [44]. The formation of heterodimers between NRP-1 and NRP-2 receptors can also link NRP-1 to Sema3C signaling [44] (Figure 2B). Although NRP-1 binds Sema3A with high affinity, this interaction cannot mediate a functional signaling cascade. The discovery of the Plexins as a binding partner of NRPs revealed that the function of NRP-1 is to bridge Sema3A to the Plexin-A1 co-receptor in order to generate a physiologically active holoreceptor complex regulating the axon guidance [45] (Figure 2C). Plexin-A1 alone does not associate with the Sema3A ligand, but the NRP-1/Plexin-A1 complex has a higher affinity and specificity for Sema3A compared with NRP-1 alone [45,46]. It is shown that Plexin-A1 activation is directly involved in the guidance of axonal growth and induced a Plexin-A1-regulated cytoskeleton collapse, causing an axon repulsion of the growth cone [41,46]. On the other hand, in the presence of a high cGMP level the Sema3A can converse the down-stream signals from repulsion to attraction in neuronal growth cone [47].
Figure 2.
Schematic presentation of the neuropilin-1 signaling complexes. The cartoon represents the complex formation between NRP-1, its ligands, and signaling co-receptors. (A) Formation of the homodimers between the NRP-1 molecules. The homodimerization of NRP-1 receptors can occur via the transmembrane domain of the proteins. The domains of the NRP-1 receptor are described in Figure 1A; (B) Heterodimerization between NRP-1 and NRP-2 receptors. The domains of the NRP-2 receptor are described in Figure 1C; (C) Structure of the functional Sema3A/NRP-1/Plexin-A1 receptor. Via this signaling complex NRP-1 is involved in the regulation of axon guidance signaling in neuronal cells and promotes repulsion signals. The complex is functionally activated upon association of NRP-1 with semaphorin 3A (Sema3A). NRP-1 a1 and a2 domains are involved in the association with Sema3A. Plexin-A1 is a single pass transmembrane protein. In the extracellular part it contains a Sema domain, a PSI motif, as Sema3A and IPT (G-P)-rich motifs. The intracellular part contains a split GAP domain separated by a GTP-ase binding domain. (D) Schematic presentation of the NRP-1 and Vascular Endothelial Growth Factor Receptor 2 (VEGFR2) holoreceptor complex. The interaction between NRP-1 receptor and VEGFR2 co-receptor is regulated via VEGF-A association, but the binding of NRP-1 to VEGFR2 enhances the signaling activity of the VEGFR2 tyrosine kinase receptor. The VEGF-A164/165 binds to the b1/b2 domains of the NRP-1 and this association enhances the VEGFR2 tyrosine autophosphorylation. VEGF-A binds to both receptors and is a crosslink between NRP-1 and VEGFR2. Via this signaling complex NRP-1 is involved the regulation of angiogenesis signaling as well as adhesion and migration of endothelial cells. (ext—extracellular environment, int—intracellular environment, GAP—GTP-ase activating protein, G-P (glycin-proline), IPT—immunoglobulin-plexin-transcription, PSI—plexin, semaphorin, integrin, VEGF—Vascular Endothelial Growth Factor, Sema3A—semaphorin 3A).
In addition to Sema3A signaling, NRP-1 plays an important role in endothelial cells via its binding to Vascular Endothelial Growth Factor A (VEGF-A), one of the most potent pro-angiogenic cytokines of the VEGF family [35,48,49]. In similarity with the nervous system in endothelial cells, NRP-1 needs the VEGFR1 or VEGFR2 signaling co-receptors to fulfill its function [24,35] (Figure 2D). VEGF receptors belong to the family of receptor tyrosine kinases and are implicated in normal embryonic development and pathological angiogenesis [50]. They can trap the VEGF ligands but the binding of VEGF-A165/VEGF-A164 to NRP-1 enhanced the VEGFR2 activity and tyrosine phosphorylation and elevated endothelial cells migration [51,52]. Currently, it is known that a strong coordination between VEGF-A, VEGFR2, and NRP-1 molecules is essential for angiogenesis [53,54], as a deletion of NRP-1 in mice failed to activate the pro-angiogenic signaling path due to defective vasculature formation [55]. Studies in human umbilical vein endothelial cells revealed that NRP-1 association with VEGFR2 is VEGF165-dependent as confirmed by co-immunoprecipitation assay [51]. Moreover, the complex between NRP-1 and the VEGFR2 can be assembled in cis (both receptors are expressed in the same cell) as well as in trans (the complex is formed between receptors present on different cells) [51]. Thus, the NRP-1 receptor could function as an extracellular scaffold molecule generating cell–cell crosstalk communications and cross-signaling.
During the last decade the involvement of NRPs in tumor biology [56] and immunology [57] was investigated. It has been reported that NRP-1 is implicated in the signaling events downstream of transforming growth factor beta (TGF-β1) [27,58,59], or platelet-derived growth factor (PDGF) [58,60] and the number of NRP-1 signaling receptor complexes is constantly growing. Many of the signaling cascades related to NRP-1 function in different cell types need to be further studied as the mechanisms are not well understood. NRP-1 was also very recently reported as a molecule able to transport other large molecules into the cells and may play a cargo role [61].
NRP-1 participates in molecular interactions through its intracellular SEA consensus domain that recruits to NRP-1 PDZ-domain containing proteins. The best characterized is synectin [40]. Synectin is involved in the arteriogenesis [62] and facilitation of the trafficking of endocytosed membrane receptors VEGFR2 and NRP-1 [49,63]. A recent study demonstrated that NRP-1 in endothelial cells regulates the focal adhesion turnover through its association with p130Cas [64].
4. NRP-1 Expression in Diabetes and Diabetic Nephropathy
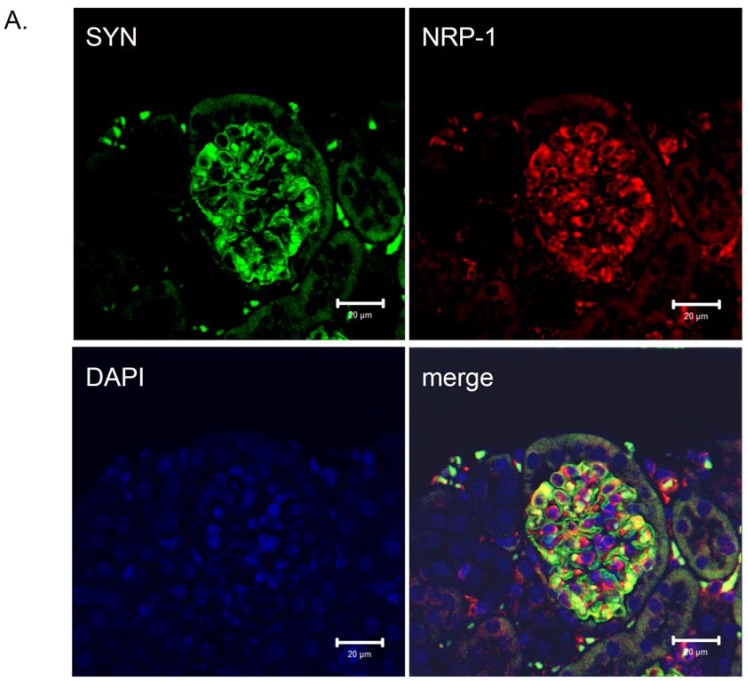
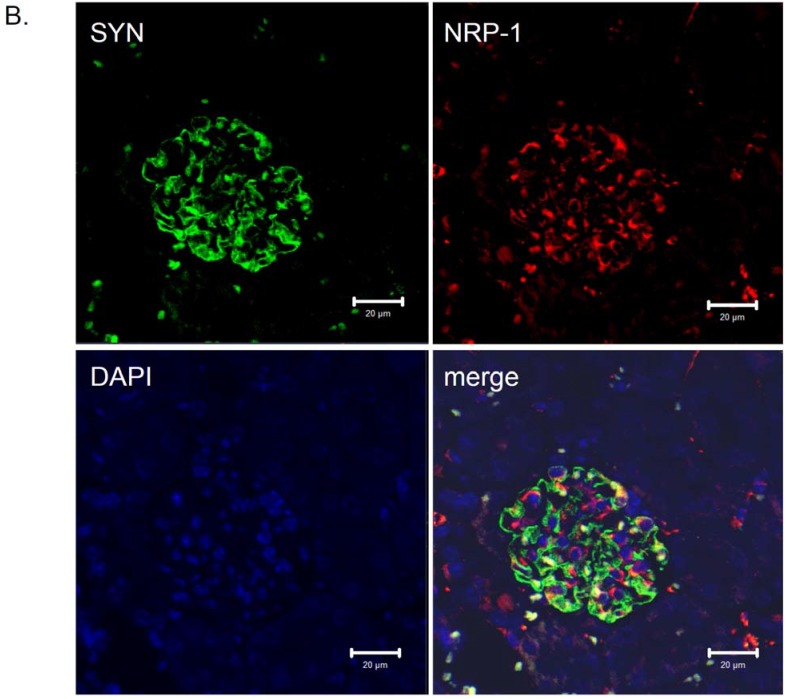
The expression of neuropilin-1 mRNA and protein in renal cells was previously described [15,16,17,18,65]. Villegas and Tufro reported that NRP-1 expression did not change in cultured undifferentiated and differentiated podocytes [18,65]. We detected that NRP-1,2 are highly expressed in differentiated podocytes [17,66]. Moreover, the immunohistochemistry staining for NRP-1 in glomeruli localized this protein to podocytes [17,67]. The presence of NRP-1 in differentiated podocytes was also confirmed in vitro and in vivo by Robert et al. [15]. Due to prolonged hyperglycemia in diabetic patients, accumulation of advanced-glycation end-products (AGEs) is highly increased [5]. Glomerular podocytes are a target of AGEs in diabetes through an elevated expression of their receptor RAGE [68] and AGEs/RAGE axis activation. Recently, we identified Nrp-1 as a downregulated gene in cultured differentiated podocytes due to glycated-BSA exposure [17,66]. Moreover, we detected a reduced NRP-1 protein expression in the glomeruli of diabetic db/db mice, an animal model to study DN [17,67] (Figure 3) (original figure) and in kidney biopsies from patients with DN [17]. A recent study demonstrated that treatment with epoetin-β or continuous erythropoietin receptor activator (CERA) of diabetic db/db mice correlated with a reduced albuminuria and increased expression of NRP-1 in treated animals compared with the non-treated [67]. These data support the observation that reduced NRP-1 expression is a characteristic of DN, and reversing/preventing the injury of podocytes is associated with a regain of NRP-1 expression [67]. Using a reporter assay analysis, we found that the regulation of NRP-1 in cultured differentiated podocytes was under the control of the Sp-1 transcription factor as mutations of the Sp-1 sites on the NRP-1 promoter completely abolished its activity [66]. Our data also confirm the findings of Rossignol et al. [69], showing a similar regulatory mechanism of the NRP-1 promoter in HeLA cells [69]. In agreement with our previous finding, glycated-BSA inhibited NRP-1 promoter activity, thus reducing the Nrp-1 gene expression in differentiated podocytes [17,66] through reduced binding of the Sp1-transcription factor to the NRP-1 promoter [66]. Furthermore, a TGF-β1 dependent downregulation of NRP-1 but not NRP-2 expression was also reported in human proteinuric nephropathies and cytokine-stimulated proximal tubular cells in a TGF-β1-dependent manner [70].
Figure 3.
Distribution of neuropilin-1 protein in kidney glomeruli of diabetic db/db mice and non-diabetic db/m littermates. Double immunological detection of neuropilin-1 (NRP-1) and synaptopodin (SYN), a podocyte specific marker protein. Nuclei were counterstained with DAPI. Images were analyzed using LSM 510 META and ZEN 2009 software (Zeiss, Germany). The staining was performed on 2 µm paraffin kidney sections originated from diabetic db/db mice and non-diabetic db/m littermates. The co-localization of the NRP-1 and synaptopodin proteins is presented as a merge image. Bars correspond to 20 µm. Magnification 400x. (A) Protein expression in glomeruli of non-diabetic db/m mice. (B) Protein expression in glomeruli of diabetic db/db mice. The expression of NRP-1 is reduced in db/db mice (B) compared with non-diabetic db/m littermates (A). The NRP-1 stain is co-localized with synaptopodin, as seen on the merged images.
5. NRP-1 Is Implicated in Podocyte Adhesion and/or Migration
In DN proteinuria develops as a result of effacement of the podocyte foot processes or podocytes loss due to apoptotic events and a nude GBM is generated [71,72]. Podocytes are often found in the urine of patients with DN [71,73], but it is difficult to characterize them as apoptotic podocytes as the cells are viable and could be further cultured in vitro [74]. Thus, it is possible that the podocyte loss is a result of the weakening of their adhesion ability to GBM. Interestingly, previously we found in in vitro studies (using cultured differentiated podocytes) that treatment of the cells with glycated BSA (AGE-BSA), or reduction of the NRP-1 expression by NRP-1 siRNA, both were associated with a reduced adhesion ability of the cells to different extracellular matrixes (ECM), e.g., collagen IV, fibronectin, laminin, all of which are characteristic for the GBM [75]. Furthermore, a forced overexpression of NRP-1 reversed the adhesion capacity of podocytes to the ECM even at the presence of glycated BSA [75]. We found also that this process was accompanied by a reduced activation of the small GTPases Rac1 and Cdc42 and was manifested in cytoskeleton dysfunction, which was NRP-1 dependent [75]. Intriguingly, a recent study in HEK 293 cells unveiled a new function of collagen IV, showing that it specifically associates with the extracellular amino-terminal region of Gpr126 adhesion receptor, which is a G-protein coupled receptor, containing the CUB (complement, Uegf, Bmp1) domain [33]. This finding raises the possibility that the reduced adhesion we observed in cultured podocytes could be explained with decreased direct association between collagen IV and NRP-1 CUB domains (see Figure 1) as a result of reduced NRP-1 protein expression [75]. Furthermore, the study of Paavola et al. [33] suggests a new function of collagen IV in the basement membranes as a signaling component. At present it is unclear how NRP-1 regulates glomerular podocytes adhesion and migration processes and which signaling receptors are involved. Indeed, in endothelial cells it was demonstrated that NRP-1 has as well a VEGFR2 independent function in the regulation of endothelial cell adhesion and spreading to fibronectin and fibrilogenesis via its cytoplasmic PDZ-containing protein binding domain and association with the GIPC1 protein [76].
6. NRP-1 Ligand Sema3A and Its Function in DN
Semaphorins are a large class of secreted axon chemorepelents that are involved in axon guidance, cell adhesion, migration, invasion, and proliferation signaling paths via interaction with their receptors NRPs and plexins [77]. The holoreceptor Neuropilin-1/Plexin-A1 mediates cellular signals specifically via its ligand semaphorin 3A (Sema3A) [45]. In the kidney, Sema3A is expressed in the developing nephrons, differentiated podocytes, and collecting tubules [18]. It was reported that Sema3A is a negative regulator of the ureteric bud branching morphogenesis [78]. Studies in Sema3a−/− transgenic mice revealed that it is essential for glomerular development, because the absence of Sema3a was associated with defects in renal vascular patterning, increased number of endothelial cells within glomerular capillaries, effaced podocytes foot processes, and development of albuminuria [79]. On the other hand, podocyte-specific Sema3a overexpression in mice resulted in renal dysfunctions, revealing severe podocyte and endothelial cell damage and/or apoptosis during organogenesis as represented by glomerular hypoplasia, impaired podocyte foot processes development, completely missing podocytes slit diaphragms, congenital proteinuria, decreased Nephrin, WT1 (Wilms tumor 1), and VEGFR2 expression [79]. Interestingly, the alteration of Sema3a expression in podocytes was not associated with the modulation of the NRP-1 receptor expression [79]. These data suggest an important function of Sema3A in vascular morphogenesis and podocytes endothelial crosstalk and the formation of the glomerular filtration barrier. Taken together, the manipulation of the Sema3a expression up- or downregulation impaired the glomerular function. Nevertheless, as NRP-1 is the main Sema3A link to its signaling unit, the Plexin-A1 co-receptor, at present the exact signaling mechanism coordinating all these processes in unclear. Interestingly, in in vitro studies using differentiated podocytes we found that suppression of NRP-1 expression using siRNA transfection induced podocytes apoptosis [17] and deletion of Sema3a in podocytes in vivo also affected podocytes survival [79]. It was also shown that an overexpression of Sema3a in podocytes in vivo was associated with glomerular disease, via deregulation of the nephrin/Plexin-A1 interaction thus linking Sema3A and Plexin-A1 to SD complexes [80].
A very recent study further elucidated the role of Sema3A in DN as the authors demonstrated that Sema3A promotes diabetic nephropathy [81,82]. They reported that mice caring a podocyte-specific gain-of-function Sema3a developed massive proteinuria and experienced declining renal function and an exacerbation of the ECM protein as laminin and collagen IV accumulation [82]. In-depth studies showed that all these processes resulted from podocytes foot process effacement and F-actin collapse and were regulated via nephrin, αVβ3 integrins, and MICAL1 association with the Plexin-A1 signaling receptor [82]. This is a very interesting observation but the direct association between the Plexin-A1 signaling co-receptor and Sema3A ligand is not demonstrated, whereas NRP-1, which is the bridge between the Sema3A and Plexin-A1 signaling receptor, interacts directly with the Plexin-1 and does not need Sema3A for this binding [45,46]. In other words, the complex between NRP-1 and Plexin-A1 already exists in the cells that are expressing both molecules and is activated via association of the NRP-1 with a Sema3A ligand. Nevertheless, collectively all these reports revealed an essential role for Sema3A/NRP-1(?)/Plexin-A1 signaling in development of DN and modulation of the complex is inducing pathological changes in the glomerular podocytes.
7. NRP-1 Ligand VEGF-A and Its Function in DN
VEGF-A is highly expressed and secreted from glomerular podocytes. It is assumed that it crosses the glomerular basement membrane (GBM) and transduces signals via its own receptor VEGF receptor 2 (VEGFR2) on endothelial cells [83]. The VEGF-A/VEGFR2 complex formation is implicated in the formation and maintenance of the glomerular filtration barrier, as genetic manipulation of the Vegf-a expression in glomeruli is associated with glomerular disease in mice [84] and a podocyte-specific deletion of Vegf-a expression is characterized by impaired recruitment of the endothelial cells into glomeruli, failure of the glomerular filtration barrier formation, and congenital nephrotic syndrome [84]. However, lately it was shown that in addition to the paracrine VEGF-A signaling in glomerulus, there is evidence for autocrine VEGF-A effects, which support podocytes’ survival [85,86,87] and also link nephrin to VEGF-A signaling in podocytes [85]. VEGFR2 expression in podocytes is somewhat contradictory, but at least in cultured differentiated podocytes Foster et al. convincingly demonstrated VEGFR2-dependent effects [85]. Interestingly, we also found that the expression of the VEGFR2 in cultured differentiated podocytes is very weak and detectable by real-time PCR but not by western blot analysis [17]. Furthermore, the treatment of cultured differentiated podocytes with glycated-BSA induced the expression of VEGFR2, while it inhibited NRP-1 expression [17].
8. Linking Neuropilin-1 to the Slit Diaphragm Proteins via CD2AP/NRP-1 Complex?
Localization of the NRP-1 protein to the slit diaphragm has not yet been demonstrated but based on the known NRP-1 interaction partners and its molecular structure we would like to present some evidence for a possible participation of the NRP-1 in this complex. Slit diaphragm is a specialized structure formed between the interdigitating individual foot processes of the glomerular podocytes to maintain a constant distance between each of the processes at the opening of the urinary space [88]. Rodewald and Karnovsky, some 30 years ago, based on their electron microscopic analysis suggested that slit diaphragm has an isoporous zipper-like structure [89]. Several proteins were reported to play an important role in the formation of this structure: nephrin was suggested to be the main structural component of the slit diaphragm [90,91,92], along with Zona occludence-1 (ZO-1) [93], P-cadherin [94], β-catenin [95], FAT1 [96,97], Neph1 [98] and CD2AP [99]. Due to its large extracellular tail, NRP-1 can be a docking place for a number of different ligands but it can also participate in many homophilic and heterophilic complexes. The main structural component of the slit diaphragm, the nephrin molecule, is suggested to be bridged to the podocytes’ cytoskeleton through its interaction with the CD2-associated protein (CD2AP) [99]. In the kidney CD2AP is mainly detected in glomerular podocytes [100]. The CD2AP knockout mice die at six to eight months of age due to development of nephrotic syndrome [101]. Detailed analyses of the CD2AP function in podocytes unveiled that it is localized in close proximity with the slit diaphragm and is associated with nephrin, as demonstrated by co-immunoprecipitation studies from a differentiated podocyte cell line [99]. In endothelial cells CD2AP is found to bind to the α3/βV integrins and form a large complex also involving NRP-1, thus providing evidences that both CD2AP and NRP-1 are linked to the extracellular matrix. Furthermore, NRP-1 molecules have the ability to form dimers from adjacent cells and it could be expected as well that due to their large extracellular part they can probably form homo- or heterodimers with molecules from the “adjacent” podocytes’ foot processes, i.e., they can “bridge” the interdigitated foot processes, as the cis- and trans-dimers for NRP-1/VEGFR2 are reported, as discussed above.
9. Conclusions
DN is associated with severe renal abnormalities inducing proteinuria resulting ultimately in glomerulosclerosis, tubulointerstitial fibrosis, and extracellular matrix. Multiple factors contribute to the development of DN. High glucose levels can modify proteins, lipids, and amino acids to generate advanced glycation end-products (AGE) which are one of the key factors involved in the onset of DN. Furthermore, elevated ANG II hypertension, oxidative stress, and increased cytokine production alter the physiological signaling, induce pathological processes, and impair the function of the renal cells. During the last decades of research a lot of knowledge was accumulated in regard to the molecular mechanisms involved in the onset and development of DN. Since progressive proteinuria is one of the main characteristics of DN, studies on glomerular podocytes received a large amount of attention because podocytes are the key component maintaining the glomerular filtration barrier via formation of the slit diaphragms and GBM in concert with glomerular endothelial cells. Due to its multidomain structure, NRP-1 is capable of binding many ligands and transducing cellular signals via its co-receptors into the cells. Its direct function in DN is not as well understood as is its function as an adhesion receptor, regulating the neuron repulsion via an association to Sema3A and Pexin-A1 signaling co-receptor in the nervous system or as an important regulator of angiogenesis in endothelial cells through interaction with VEGF-A164/165 and VEGFR2 co-receptor complex. In cultured differentiated podocytes NRP-1 expression is suppressed during the exposure to AGEs, a key factor known to induce pathophysiological changes of DN. Furthermore, NRP-1 reduction was at least in part associated with a declined migration and adhesion ability of the podocytes to GBM extracellular matrix components such as collagen IV, fibronectin, and laminin, impaired cytoskeleton reorganization, podocytes apoptosis, and a lower GTP-binding activity of the small Rho-GTPases Rac1 and Cdc42. NRP-1 expression was also decreased in podocytes from diabetic db/db mice as well as in diabetic patients diagnosed with DN. Interestingly, epoetin-β and CERA treatment of db/db mice, which reduced proteinuria, also increased the NRP-1 protein expression in podocytes. Thus, the generation of podocyte-specific deletion and overexpression of NRP-1 using animal models will shed more light onto the role of NRP-1 in diabetic disease and particularly in the development of DN.
Acknowledgments
The authors thank to M. Liebisch and S. Franke for the helpful discussions.
Author Contributions
Tzvetanka Bondeva and Gunter Wolf designed the structure of the manuscript; Tzvetanka Bondeva and Gunter Wolf drafted the manuscript; Tzvetanka Bondeva and Gunter Wolf prepared the figures; Tzvetanka Bondeva and Gunter Wolf approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Wolf G., Ziyadeh F.N. Cellular and molecular mechanisms of proteinuria in diabetic nephropathy. Nephron Physiol. 2007;106:26–31. doi: 10.1159/000101797. [DOI] [PubMed] [Google Scholar]
- 2.Arora M.K., Singh U.K. Molecular mechanisms in the pathogenesis of diabetic nephropathy: An update. Vasc. Pharmacol. 2013;58:259–271. doi: 10.1016/j.vph.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Raptis A.E., Viberti G. Pathogenesis of diabetic nephropathy. Exp. Clin. Endocrinol. Diabetes. 2001;109(Suppl. S2):424–437. doi: 10.1055/s-2001-18600. [DOI] [PubMed] [Google Scholar]
- 4.Bohlender J.M., Franke S., Stein G., Wolf G. Advanced glycation end products and the kidney. Am. J. Physiol. Ren. Physiol. 2005;289:F645–F659. doi: 10.1152/ajprenal.00398.2004. [DOI] [PubMed] [Google Scholar]
- 5.Busch M., Franke S., Ruster C., Wolf G. Advanced glycation end-products and the kidney. Eur. J. Clin. Investig. 2010;40:742–755. doi: 10.1111/j.1365-2362.2010.02317.x. [DOI] [PubMed] [Google Scholar]
- 6.Heidland A., Sebekova K., Schinzel R. Advanced glycation end products and the progressive course of renal disease. Am. J. Kidney Dis. 2001;38(Suppl. S1):100–106. doi: 10.1053/ajkd.2001.27414. [DOI] [PubMed] [Google Scholar]
- 7.Wolf G. Angiotensin II as a renal growth factor. Contrib. Nephrol. 2001;135:92–110. doi: 10.1159/000060159. [DOI] [PubMed] [Google Scholar]
- 8.Chen S., Wolf G., Ziyadeh F.N. The renin-angiotensin system in diabetic nephropathy. Contrib. Nephrol. 2001;135:212–221. doi: 10.1159/000060167. [DOI] [PubMed] [Google Scholar]
- 9.Tsuchida K., Cronin B., Sharma K. Novel aspects of transforming growth factor-beta in diabetic kidney disease. Nephron. 2002;92:7–21. doi: 10.1159/000064486. [DOI] [PubMed] [Google Scholar]
- 10.Chen S., Lee J.S., Iglesias-de la Cruz M.C., Wang A., Izquierdo-Lahuerta A., Gandhi N.K., Danesh F.R., Wolf G., Ziyadeh F.N. Angiotensin ii stimulates alpha3(iv) collagen production in mouse podocytes via tgf-beta and vegf signalling: Implications for diabetic glomerulopathy. Nephrol. Dial. Transplant. 2005;20:1320–1328. doi: 10.1093/ndt/gfh837. [DOI] [PubMed] [Google Scholar]
- 11.Li J.J., Kwak S.J., Jung D.S., Kim J.J., Yoo T.H., Ryu D.R., Han S.H., Choi H.Y., Lee J.E., Moon S.J., et al. Podocyte biology in diabetic nephropathy. Kidney Int. Suppl. 2007:S36–S42. doi: 10.1038/sj.ki.5002384. [DOI] [PubMed] [Google Scholar]
- 12.Pellet-Many C., Frankel P., Jia H., Zachary I. Neuropilins: Structure, function and role in disease. Biochem. J. 2008;411:211–226. doi: 10.1042/BJ20071639. [DOI] [PubMed] [Google Scholar]
- 13.Takagi S., Kasuya Y., Shimizu M., Matsuura T., Tsuboi M., Kawakami A., Fujisawa H. Expression of a cell adhesion molecule, neuropilin, in the developing chick nervous system. Dev. Biol. 1995;170:207–222. doi: 10.1006/dbio.1995.1208. [DOI] [PubMed] [Google Scholar]
- 14.Chen H., Chedotal A., He Z., Goodman C.S., Tessier-Lavigne M. Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins sema e and sema iv but not sema iii. Neuron. 1997;19:547–559. doi: 10.1016/S0896-6273(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 15.Robert B., Zhao X., Abrahamson D.R. Coexpression of neuropilin-1, flk1, and vegf(164) in developing and mature mouse kidney glomeruli. Am. J. Physiol. Ren. Physiol. 2000;279:F275–F282. doi: 10.1152/ajprenal.2000.279.2.F275. [DOI] [PubMed] [Google Scholar]
- 16.Harper S.J., Xing C.Y., Whittle C., Parry R., Gillatt D., Peat D., Mathieson P.W. Expression of neuropilin-1 by human glomerular epithelial cells in vitro and in vivo. Clin. Sci. (Lond.) 2001;101:439–446. doi: 10.1042/CS20010025. [DOI] [PubMed] [Google Scholar]
- 17.Bondeva T., Ruster C., Franke S., Hammerschmid E., Klagsbrun M., Cohen C.D., Wolf G. Advanced glycation end-products suppress neuropilin-1 expression in podocytes. Kidney Int. 2009;75:605–616. doi: 10.1038/ki.2008.603. [DOI] [PubMed] [Google Scholar]
- 18.Villegas G., Tufro A. Ontogeny of semaphorins 3a and 3f and their receptors neuropilins 1 and 2 in the kidney. Mech. Dev. 2002;119(Suppl. S1):149–153. doi: 10.1016/S0925-4773(03)00108-4. [DOI] [PubMed] [Google Scholar]
- 19.Takagi S., Hirata T., Agata K., Mochii M., Eguchi G., Fujisawa H. The a5 antigen, a candidate for the neuronal recognition molecule, has homologies to complement components and coagulation factors. Neuron. 1991;7:295–307. doi: 10.1016/0896-6273(91)90268-5. [DOI] [PubMed] [Google Scholar]
- 20.He Z., Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent semaphorin iii. Cell. 1997;90:739–751. doi: 10.1016/S0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- 21.Kolodkin A.L., Levengood D.V., Rowe E.G., Tai Y.T., Giger R.J., Ginty D.D. Neuropilin is a semaphorin iii receptor. Cell. 1997;90:753–762. doi: 10.1016/S0092-8674(00)80535-8. [DOI] [PubMed] [Google Scholar]
- 22.Reza J.N., Gavazzi I., Cohen J. Neuropilin-1 is expressed on adult mammalian dorsal root ganglion neurons and mediates semaphorin3a/collapsin-1-induced growth cone collapse by small diameter sensory afferents. Mol. Cell. Neurosci. 1999;14:317–326. doi: 10.1006/mcne.1999.0786. [DOI] [PubMed] [Google Scholar]
- 23.Li F., Zhao H., Liao Y., Takashima S., Asano Y., Shintani Y., Hori M., Kitakaze M. Higher mortality in heterozygous neuropilin-1 mice after cardiac pressure overload. Biochem. Biophys. Res. Commun. 2008;370:317–321. doi: 10.1016/j.bbrc.2008.03.096. [DOI] [PubMed] [Google Scholar]
- 24.Soker S., Takashima S., Miao H.Q., Neufeld G., Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/S0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 25.Miao H.Q., Lee P., Lin H., Soker S., Klagsbrun M. Neuropilin-1 expression by tumor cells promotes tumor angiogenesis and progression. FASEB J. 2000;14:2532–2539. doi: 10.1096/fj.00-0250com. [DOI] [PubMed] [Google Scholar]
- 26.Tordjman R., Ortega N., Coulombel L., Plouet J., Romeo P.H., Lemarchandel V. Neuropilin-1 is expressed on bone marrow stromal cells: A novel interaction with hematopoietic cells? Blood. 1999;94:2301–2309. [PubMed] [Google Scholar]
- 27.Glinka Y., Prud’homme G.J. Neuropilin-1 is a receptor for transforming growth factor beta-1, activates its latent form, and promotes regulatory t cell activity. J. Leukoc. Biol. 2008;84:302–310. doi: 10.1189/jlb.0208090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarris M., Andersen K.G., Randow F., Mayr L., Betz A.G. Neuropilin-1 expression on regulatory t cells enhances their interactions with dendritic cells during antigen recognition. Immunity. 2008;28:402–413. doi: 10.1016/j.immuni.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kardos J., Gal P., Szilagyi L., Thielens N.M., Szilagyi K., Lorincz Z., Kulcsar P., Graf L., Arlaud G.J., Zavodszky P. The role of the individual domains in the structure and function of the catalytic region of a modular serine protease, c1r. J. Immunol. 2001;167:5202–5208. doi: 10.4049/jimmunol.167.9.5202. [DOI] [PubMed] [Google Scholar]
- 30.Delgadillo-Reynoso M.G., Rollo D.R., Hursh D.A., Raff R.A. Structural analysis of the uegf gene in the sea urchin strongylocentrotus purpuratus reveals more similarity to vertebrate than to invertebrate genes with egf-like repeats. J. Mol. Evol. 1989;29:314–327. doi: 10.1007/BF02103619. [DOI] [PubMed] [Google Scholar]
- 31.Hartigan N., Garrigue-Antar L., Kadler K.E. Bone morphogenetic protein-1 (bmp-1). Identification of the minimal domain structure for procollagen c-proteinase activity. J. Biol. Chem. 2003;278:18045–18049. doi: 10.1074/jbc.M211448200. [DOI] [PubMed] [Google Scholar]
- 32.Orchard-Webb D.J., Lee T.C., Cook G.P.M., Blair G.E. Cub domain containing protein 1 (cdcp1) modulates adhesion and motility in colon cancer cells. BMC Cancer. 2014;14:754. doi: 10.1186/1471-2407-14-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paavola K.J., Sidik H., Zuchero J.B., Eckart M., Talbot W.S. Type iv collagen is an activating ligand for the adhesion g protein-coupled receptor gpr126. Sci. Signal. 2014;7:ra76. doi: 10.1126/scisignal.2005347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Appleton B.A., Wu P., Maloney J., Yin J., Liang W.C., Stawicki S., Mortara K., Bowman K.K., Elliott J.M., Desmarais W., et al. Structural studies of neuropilin/antibody complexes provide insights into semaphorin and vegf binding. EMBO J. 2007;26:4902–4912. doi: 10.1038/sj.emboj.7601906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parker M.W., Xu P., Guo H.F., Vander Kooi C.W. Mechanism of selective vegf-a binding by neuropilin-1 reveals a basis for specific ligand inhibition. PLoS ONE. 2012;7:e49177. doi: 10.1371/journal.pone.0049177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaushal G.P., Haun R.S., Herzog C., Shah S.V. Meprin a metalloproteinase and its role in acute kidney injury. Am. J. Physiol. Ren. Physiol. 2013;304:F1150–F1158. doi: 10.1152/ajprenal.00014.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aricescu A.R., Hon W.C., Siebold C., Lu W., van der Merwe P.A., Jones E.Y. Molecular analysis of receptor protein tyrosine phosphatase mu-mediated cell adhesion. EMBO J. 2006;25:701–712. doi: 10.1038/sj.emboj.7600974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cismasiu V.B., Denes S.A., Reilander H., Michel H., Szedlacsek S.E. The mam (meprin/a5-protein/ptpmu) domain is a homophilic binding site promoting the lateral dimerization of receptor-like protein-tyrosine phosphatase mu. J. Biol. Chem. 2004;279:26922–26931. doi: 10.1074/jbc.M313115200. [DOI] [PubMed] [Google Scholar]
- 39.Roth L., Nasarre C., Dirrig-Grosch S., Aunis D., Cremel G., Hubert P., Bagnard D. Transmembrane domain interactions control biological functions of neuropilin-1. Mol. Biol. Cell. 2008;19:646–654. doi: 10.1091/mbc.E07-06-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cai H., Reed R.R. Cloning and characterization of neuropilin-1-interacting protein: A psd-95/dlg/zo-1 domain-containing protein that interacts with the cytoplasmic domain of neuropilin-1. J. Neurosci. 1999;19:6519–6527. doi: 10.1523/JNEUROSCI.19-15-06519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parker M.W., Guo H.F., Li X., Linkugel A.D., Vander Kooi C.W. Function of members of the neuropilin family as essential pleiotropic cell surface receptors. Biochemistry. 2012;51:9437–9446. doi: 10.1021/bi3012143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwarz Q., Ruhrberg C. Neuropilin, you gotta let me know: Should i stay or should i go? Cell Adhes. Migr. 2010;4:61–66. doi: 10.4161/cam.4.1.10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakamura F., Tanaka M., Takahashi T., Kalb R.G., Strittmatter S.M. Neuropilin-1 extracellular domains mediate semaphorin d/iii-induced growth cone collapse. Neuron. 1998;21:1093–1100. doi: 10.1016/S0896-6273(00)80626-1. [DOI] [PubMed] [Google Scholar]
- 44.Giger R.J., Urquhart E.R., Gillespie S.K., Levengood D.V., Ginty D.D., Kolodkin A.L. Neuropilin-2 is a receptor for semaphorin iv: Insight into the structural basis of receptor function and specificity. Neuron. 1998;21:1079–1092. doi: 10.1016/S0896-6273(00)80625-X. [DOI] [PubMed] [Google Scholar]
- 45.Rohm B., Ottemeyer A., Lohrum M., Puschel A.W. Plexin/neuropilin complexes mediate repulsion by the axonal guidance signal semaphorin 3a. Mech. Dev. 2000;93:95–104. doi: 10.1016/S0925-4773(00)00269-0. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi T., Fournier A., Nakamura F., Wang L.H., Murakami Y., Kalb R.G., Fujisawa H., Strittmatter S.M. Plexin-neuropilin-1 complexes form functional semaphorin-3a receptors. Cell. 1999;99:59–69. doi: 10.1016/S0092-8674(00)80062-8. [DOI] [PubMed] [Google Scholar]
- 47.Song H., Ming G., He Z., Lehmann M., McKerracher L., Tessier-Lavigne M., Poo M. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- 48.Lampropoulou A., Ruhrberg C. Neuropilin regulation of angiogenesis. Biochem. Soc. Trans. 2014;42:1623–1628. doi: 10.1042/BST20140244. [DOI] [PubMed] [Google Scholar]
- 49.Lanahan A., Zhang X., Fantin A., Zhuang Z., Rivera-Molina F., Speichinge K., Prahst C., Zhang J., Wang Y., Davis G., et al. The neuropilin 1 cytoplasmic domain is required for vegf-a-dependent arteriogenesis. Dev. Cell. 2013;25:156–168. doi: 10.1016/j.devcel.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rahimi N. Vascular endothelial growth factor receptors: Molecular mechanisms of activation and therapeutic potentials. Exp. Eye Res. 2006;83:1005–1016. doi: 10.1016/j.exer.2006.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Soker S., Miao H.Q., Nomi M., Takashima S., Klagsbrun M. Vegf165 mediates formation of complexes containing vegfr-2 and neuropilin-1 that enhance vegf165-receptor binding. J. Cell. Biochem. 2002;85:357–368. doi: 10.1002/jcb.10140. [DOI] [PubMed] [Google Scholar]
- 52.Herzog B., Pellet-Many C., Britton G., Hartzoulakis B., Zachary I.C. Vegf binding to nrp1 is essential for vegf stimulation of endothelial cell migration, complex formation between nrp1 and vegfr2, and signaling via fak tyr407 phosphorylation. Mol. Biol. Cell. 2011;22:2766–2776. doi: 10.1091/mbc.E09-12-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zachary I.C. How neuropilin-1 regulates receptor tyrosine kinase signalling: The knowns and known unknowns. Biochem. Soc. Trans. 2011;39:1583–1591. doi: 10.1042/BST20110697. [DOI] [PubMed] [Google Scholar]
- 54.Klagsbrun M., Takashima S., Mamluk R. The role of neuropilin in vascular and tumor biology. Adv. Exp. Med. Biol. 2002;515:33–48. doi: 10.1007/978-1-4615-0119-0_3. [DOI] [PubMed] [Google Scholar]
- 55.Jones E.A., Yuan L., Breant C., Watts R.J., Eichmann A. Separating genetic and hemodynamic defects in neuropilin 1 knockout embryos. Development. 2008;135:2479–2488. doi: 10.1242/dev.014902. [DOI] [PubMed] [Google Scholar]
- 56.Prud’homme G.J., Glinka Y. Neuropilins are multifunctional coreceptors involved in tumor initiation, growth, metastasis and immunity. Oncotarget. 2012;3:921–939. doi: 10.18632/oncotarget.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumanogoh A., Kikutani H. Immunological functions of the neuropilins and plexins as receptors for semaphorins. Nat. Rev. Immunol. 2013;13:802–814. doi: 10.1038/nri3545. [DOI] [PubMed] [Google Scholar]
- 58.Pellet-Many C., Frankel P., Evans I.M., Herzog B.M., Junemann-Ramirez M., Zachary I.C. Neuropilin-1 mediates pdgf stimulation of vascular smooth muscle cell migration and signalling via p130cas. Biochem. J. 2011;435:609–618. doi: 10.1042/BJ20100580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glinka Y., Stoilova S., Mohammed N., Prud’homme G.J. Neuropilin-1 exerts co-receptor function for tgf-beta-1 on the membrane of cancer cells and enhances responses to both latent and active tgf-beta. Carcinogenesis. 2011;32:613–621. doi: 10.1093/carcin/bgq281. [DOI] [PubMed] [Google Scholar]
- 60.Ball S.G., Shuttleworth C.A., Kielty C.M. Platelet-derived growth factor receptors regulate mesenchymal stem cell fate: Implications for neovascularization. Expert Opin. Biol. Ther. 2010;10:57–71. doi: 10.1517/14712590903379510. [DOI] [PubMed] [Google Scholar]
- 61.Teesalu T., Sugahara K.N., Kotamraju V.R., Ruoslahti E. C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc. Natl. Acad. Sci. USA. 2009;106:16157–16162. doi: 10.1073/pnas.0908201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang L., Mukhopadhyay D., Xu X. C terminus of rgs-gaip-interacting protein conveys neuropilin-1-mediated signaling during angiogenesis. FASEB J. 2006;20:1513–1515. doi: 10.1096/fj.05-5504fje. [DOI] [PubMed] [Google Scholar]
- 63.Naccache S.N., Hasson T. Myosin vi altered at threonine 406 stabilizes actin filaments in vivo. Cell Motil. Cytoskeleton. 2006;63:633–645. doi: 10.1002/cm.20150. [DOI] [PubMed] [Google Scholar]
- 64.Seerapu H.R., Borthakur S., Kong N., Agrawal S., Drazba J., Vasanji A., Fantin A., Ruhrberg C., Buck M., Horowitz A. The cytoplasmic domain of neuropilin-1 regulates focal adhesion turnover. FEBS Lett. 2013;587:3392–3399. doi: 10.1016/j.febslet.2013.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guan F., Villegas G., Teichman J., Mundel P., Tufro A. Autocrine class 3 semaphorin system regulates slit diaphragm proteins and podocyte survival. Kidney Int. 2006;69:1564–1569. doi: 10.1038/sj.ki.5000313. [DOI] [PubMed] [Google Scholar]
- 66.Bondeva T., Wolf G. Advanced glycation end products suppress neuropilin-1 expression in podocytes by a reduction in sp1-dependent transcriptional activity. Am. J. Nephrol. 2009;30:336–345. doi: 10.1159/000227762. [DOI] [PubMed] [Google Scholar]
- 67.Loeffler I., Ruster C., Franke S., Liebisch M., Wolf G. Erythropoietin ameliorates podocyte injury in advanced diabetic nephropathy in the db/db mouse. Am. J. Physiol. Ren. Physiol. 2013;305:F911–F918. doi: 10.1152/ajprenal.00643.2012. [DOI] [PubMed] [Google Scholar]
- 68.Wendt T.M., Tanji N., Guo J., Kislinger T.R., Qu W., Lu Y., Bucciarelli L.G., Rong L.L., Moser B., Markowitz G.S., et al. Rage drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy. Am. J. Pathol. 2003;162:1123–1137. doi: 10.1016/S0002-9440(10)63909-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rossignol M., Pouyssegur J., Klagsbrun M. Characterization of the neuropilin-1 promoter; gene expression is mediated by the transcription factor sp1. J. Cell. Biochem. 2003;88:744–757. doi: 10.1002/jcb.10384. [DOI] [PubMed] [Google Scholar]
- 70.Schramek H., Sarkozi R., Lauterberg C., Kronbichler A., Pirklbauer M., Albrecht R., Noppert S.J., Perco P., Rudnicki M., Strutz F.M., et al. Neuropilin-1 and neuropilin-2 are differentially expressed in human proteinuric nephropathies and cytokine-stimulated proximal tubular cells. Lab. Investig. 2009;89:1304–1316. doi: 10.1038/labinvest.2009.96. [DOI] [PubMed] [Google Scholar]
- 71.Meyer T.W., Bennett P.H., Nelson R.G. Podocyte number predicts long-term urinary albumin excretion in pima indians with type ii diabetes and microalbuminuria. Diabetologia. 1999;42:1341–1344. doi: 10.1007/s001250051447. [DOI] [PubMed] [Google Scholar]
- 72.Pagtalunan M.E., Miller P.L., Jumping-Eagle S., Nelson R.G., Myers B.D., Rennke H.G., Coplon N.S., Sun L., Meyer T.W. Podocyte loss and progressive glomerular injury in type ii diabetes. J. Clin. Investig. 1997;99:342–348. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakamura T., Ushiyama C., Suzuki S., Hara M., Shimad N., Ebihara I., Koide H. Urinary excretion of podocytes in patients with diabetic nephropathy. Nephrol. Dial. Transplant. 2000;15:1379–1383. doi: 10.1093/ndt/15.9.1379. [DOI] [PubMed] [Google Scholar]
- 74.Petermann A.T., Pippin J., Krofft R., Blonski M., Griffin S., Durvasula R., Shankland S.J. Viable podocytes detach in experimental diabetic nephropathy: Potential mechanism underlying glomerulosclerosis. Nephron Exp. Nephrol. 2004;98 doi: 10.1159/000081555. [DOI] [PubMed] [Google Scholar]
- 75.Bondeva T., Wojciech S., Wolf G. Advanced glycation end products inhibit adhesion ability of differentiated podocytes in a neuropilin-1-dependent manner. Am. J. Physiol. Ren. Physiol. 2011;301 doi: 10.1152/ajprenal.00575.2010. [DOI] [PubMed] [Google Scholar]
- 76.Valdembri D., Caswell P.T., Anderson K.I., Schwarz J.P., Konig I., Astanina E., Caccavari F., Norman J.C., Humphries M.J., Bussolino F., et al. Neuropilin-1/gipc1 signaling regulates alpha5beta1 integrin traffic and function in endothelial cells. PLoS Biol. 2009;7:e25. doi: 10.1371/journal.pbio.1000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Staton C.A. Class 3 semaphorins and their receptors in physiological and pathological angiogenesis. Biochem. Soc. Trans. 2011;39:1565–1570. doi: 10.1042/BST20110654. [DOI] [PubMed] [Google Scholar]
- 78.Tufro A., Teichman J., Woda C., Villegas G. Semaphorin3a inhibits ureteric bud branching morphogenesis. Mech. Dev. 2008;125:558–568. doi: 10.1016/j.mod.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reidy K.J., Villegas G., Teichman J., Veron D., Shen W., Jimenez J., Thomas D., Tufro A. Semaphorin3a regulates endothelial cell number and podocyte differentiation during glomerular development. Development. 2009;136:3979–3989. doi: 10.1242/dev.037267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reidy K.J., Aggarwal P.K., Jimenez J.J., Thomas D.B., Veron D., Tufro A. Excess podocyte semaphorin-3a leads to glomerular disease involving plexina1-nephrin interaction. Am. J. Pathol. 2013;183:1156–1168. doi: 10.1016/j.ajpath.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Reidy K., Tufro A. Semaphorins in kidney development and disease: Modulators of ureteric bud branching, vascular morphogenesis, and podocyte-endothelial crosstalk. Pediatr. Nephrol. 2011;26:1407–1412. doi: 10.1007/s00467-011-1769-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aggarwal P.K., Veron D., Thomas D.B., Siegel D., Moeckel G., Kashgarian M., Tufro A. Semaphorin3a promotes advanced diabetic nephropathy. Diabetes. 2015;64:1743–1759. doi: 10.2337/db14-0719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Eremina V., Baelde H.J., Quaggin S.E. Role of the vegf—A signaling pathway in the glomerulus: Evidence for crosstalk between components of the glomerular filtration barrier. Nephron Physiol. 2007;106:32–37. doi: 10.1159/000101798. [DOI] [PubMed] [Google Scholar]
- 84.Eremina V., Sood M., Haigh J., Nagy A., Lajoie G., Ferrara N., Gerber H.P., Kikkawa Y., Miner J.H., Quaggin S.E. Glomerular-specific alterations of vegf-a expression lead to distinct congenital and acquired renal diseases. J. Clin. Investig. 2003;111:707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Foster R.R., Saleem M.A., Mathieson P.W., Bates D.O., Harper S.J. Vascular endothelial growth factor and nephrin interact and reduce apoptosis in human podocytes. Am. J. Physiol. Ren. Physiol. 2005;288:48–57. doi: 10.1152/ajprenal.00146.2004. [DOI] [PubMed] [Google Scholar]
- 86.Foster R.R., Hole R., Anderson K., Satchell S.C., Coward R.J., Mathieson P.W., Gillatt D.A., Saleem M.A., Bates D.O., Harper S.J. Functional evidence that vascular endothelial growth factor may act as an autocrine factor on human podocytes. Am. J. Physiol. Ren. Physiol. 2003;284:1263–1273. doi: 10.1152/ajprenal.00276.2002. [DOI] [PubMed] [Google Scholar]
- 87.Guan F., Villegas G., Teichman J., Mundel P., Tufro A. Autocrine vegf-a system in podocytes regulates podocin and its interaction with cd2ap. Am. J. Physiol. Ren. Physiol. 2006;291:422–428. doi: 10.1152/ajprenal.00448.2005. [DOI] [PubMed] [Google Scholar]
- 88.Karnovsky M.J., Ainsworth S.K. The structural basis of glomerular filtration. Adv. Nephrol. Necker Hosp. 1972;2:35–60. [PubMed] [Google Scholar]
- 89.Rodewald R., Karnovsky M.J. Porous substructure of the glomerular slit diaphragm in the rat and mouse. J. Cell Biol. 1974;60:423–433. doi: 10.1083/jcb.60.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Patrakka J., Tryggvason K. Nephrin—A unique structural and signaling protein of the kidney filter. Trends Mol. Med. 2007;13:396–403. doi: 10.1016/j.molmed.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 91.Ruotsalainen V., Ljungberg P., Wartiovaara J., Lenkkeri U., Kestila M., Jalanko H., Holmberg C., Tryggvason K. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc. Natl. Acad. Sci. USA. 1999;96:7962–7967. doi: 10.1073/pnas.96.14.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Khoshnoodi J., Sigmundsson K., Ofverstedt L.G., Skoglund U., Obrink B., Wartiovaara J., Tryggvason K. Nephrin promotes cell-cell adhesion through homophilic interactions. Am. J. Pathol. 2003;163:2337–2346. doi: 10.1016/S0002-9440(10)63590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schnabel E., Anderson J.M., Farquhar M.G. The tight junction protein zo-1 is concentrated along slit diaphragms of the glomerular epithelium. J. Cell Biol. 1990;111:1255–1263. doi: 10.1083/jcb.111.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu Z.G., Ryu D.R., Yoo T.H., Jung D.S., Kim J.J., Kim H.J., Choi H.Y., Kim J.S., Adler S.G., Natarajan R., et al. P-cadherin is decreased in diabetic glomeruli and in glucose-stimulated podocytes in vivo and in vitro studies. Nephrol. Dial. Transplant. 2005;20:524–531. doi: 10.1093/ndt/gfh642. [DOI] [PubMed] [Google Scholar]
- 95.Reiser J., Kriz W., Kretzler M., Mundel P. The glomerular slit diaphragm is a modified adherens junction. J. Am. Soc. Nephrol. 2000;11:1–8. doi: 10.1681/ASN.V1111. [DOI] [PubMed] [Google Scholar]
- 96.Ciani L., Patel A., Allen N.D., ffrench-Constant C. Mice lacking the giant protocadherin mfat1 exhibit renal slit junction abnormalities and a partially penetrant cyclopia and anophthalmia phenotype. Mol. Cell. Biol. 2003;23:3575–3582. doi: 10.1128/MCB.23.10.3575-3582.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Inoue T., Yaoita E., Kurihara H., Shimizu F., Sakai T., Kobayashi T., Ohshiro K., Kawachi H., Okada H., Suzuki H., et al. Fat is a component of glomerular slit diaphragms. Kidney Int. 2001;59:1003–1012. doi: 10.1046/j.1523-1755.2001.0590031003.x. [DOI] [PubMed] [Google Scholar]
- 98.Donoviel D.B., Freed D.D., Vogel H., Potter D.G., Hawkins E., Barrish J.P., Mathur B.N., Turner C.A., Geske R., Montgomery C.A., et al. Proteinuria and perinatal lethality in mice lacking neph1, a novel protein with homology to nephrin. Mol. Cell. Biol. 2001;21:4829–4836. doi: 10.1128/MCB.21.14.4829-4836.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shih N.Y., Li J., Cotran R., Mundel P., Miner J.H., Shaw A.S. Cd2ap localizes to the slit diaphragm and binds to nephrin via a novel c-terminal domain. Am. J. Pathol. 2001;159:2303–2308. doi: 10.1016/S0002-9440(10)63080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wolf G., Stahl R.A. CD2-associated protein and glomerular disease. Lancet. 2003;362:1746–1748. doi: 10.1016/S0140-6736(03)14856-8. [DOI] [PubMed] [Google Scholar]
- 101.Shih N.Y., Li J., Karpitskii V., Nguyen A., Dustin M.L., Kanagawa O., Miner J.H., Shaw A.S. Congenital nephrotic syndrome in mice lacking cd2-associated protein. Science. 1999;286:312–315. doi: 10.1126/science.286.5438.312. [DOI] [PubMed] [Google Scholar]