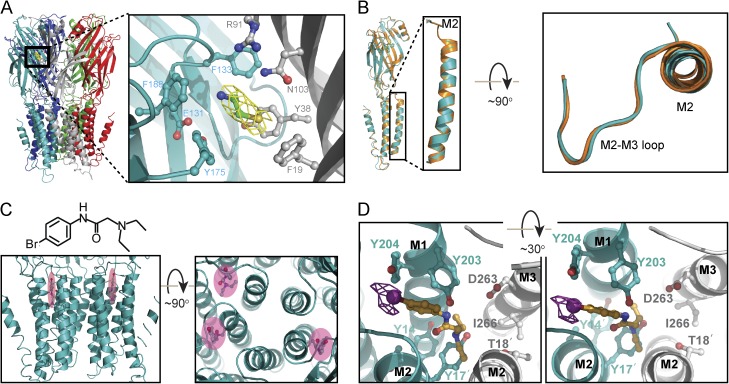
Figure 7.
X-ray crystal structure of wild-type ELIC bound to cysteamine and either lidocaine or a brominated analogue. (A) Pentameric architecture of ELIC. The inset is a magnified view of the cysteamine-binding site with surrounding side chains shown in ball-and-stick representation. The carbon atoms of the amino acids forming the primary and secondary interfaces are represented in teal and gray, respectively. The yellow mesh represents the 2Fo–Fc electron-density map of cysteamine (carbon atoms, in green) contoured at the level of 1σ. The atoms of oxygen are shown in red; those of nitrogen, in blue; and those of sulfur, in yellow. (B) Structural alignment of wild-type ELIC cocrystalized with cysteamine and lidocaine (teal) with the previously solved model of unliganded wild-type ELIC (PDB code: 2VL0; orange); no change is apparent. For clarity, only one subunit is shown. The inset is a magnified view of the M2 α-helix and the M2–M3 loop. (C) Lateral and top views of the binding site for the bromo derivative N-1-(4-bromophenyl)-N-2,N-2 diethyl glycinamide (shadowed in pink). (D) A magnified view (from the extracellular side) of the bromo-derivative binding site; only two subunits are shown. Protein and brominated-analogue atoms are colored as in A, with the exception of the carbon atoms of the latter, which are shown in orange; the bromine atom is shown in purple. The anomalous-difference map corresponding to the bromo derivative (calculated at 4.8 Å and contoured at 4.5σ) is also displayed in purple. The amino acids in the M2 α-helices are denoted using the prime notation.