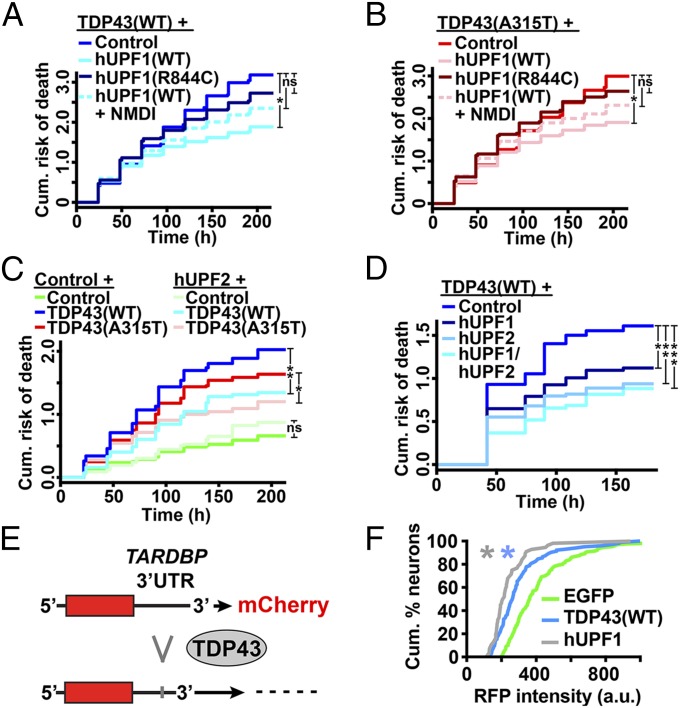
Fig. 5.
Neuroprotection by hUPF1 requires helicase activity and involves NMD. hUPF1 improved survival in cells expressing TDP43(WT) (A) and TDP43(A315T) (B), but helicase-null hUPF1(R844C) was ineffective, and rescue was attenuated with the addition of NMDI-1, an inhibitor of NMD. (C) hUPF2 also improved survival in neurons expressing TDP43(WT) and TDP43(A315T). (D) Expression of both hUPF1 and hUPF2 further reduced the risk of death in neurons transfected with TDP43(WT). In A and B, *P < 0.01; in C, *P = 0.02, **P ≤ 1 × 10−4; in D, *P = 0.02, **P < 0.001, ***P < 1 × 10−5; ns, not significant by Cox hazards analysis. In A and B, n = 360–686 neurons per genotype. In C and D, n = 87–258 neurons per genotype. Results were pooled from eight wells per condition, in three or more experiments. (E) Binding of TDP43 to the TARDBP 3′UTR triggers splicing and RNA decay of a fluorescent reporter. (F) TDP43(WT) (n = 104) and hUPF1 (n = 56) effectively reduce single-cell reporter intensity, compared with EGFP (n = 130). *P ≤ 0.05, Kolmogorov–Smirnov test. Results pooled from 8–16 wells per experiment, in duplicate.