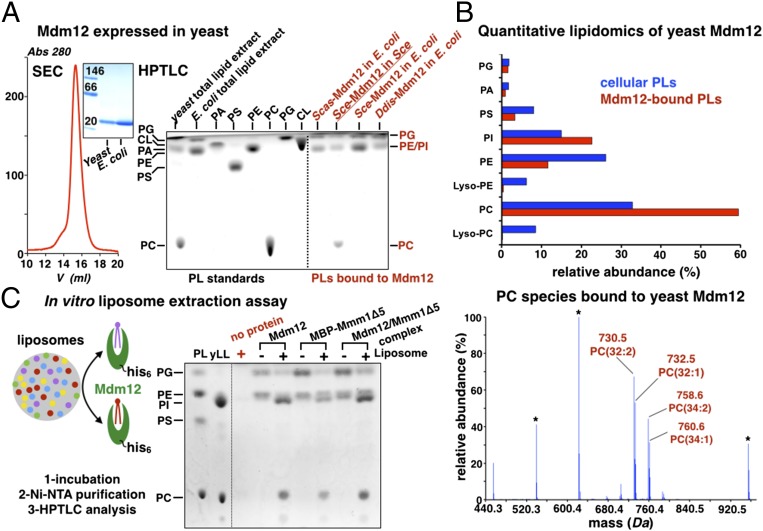
Fig. 6.
Phosphatidylcholines are bona fide ligands of Mdm12 and Mmm1. (A) Yeast Mdm12 purified from yeast is monomeric and binds PC, PE, and PI. SEC profile of Mdm12 purified from yeast. In BN-PAGE of yeast purified from yeast or E. coli, the two proteins are monomeric and undistinguishable. HPTLC analyses of three different Mdm12 proteins from S. cerevisiae (Sce), S. castellii (Scas), and the amoeba D. discoideum (Ddis). All three proteins expressed in E. coli contain PE and PG. Mdm12 purified from its native source (yeast) contains PC, PE, and PI. (B) Lipid profiling by ESI-MS of yeast Mdm12 purified from yeast shows that Mdm12 preferentially binds to PCs in vivo. (Upper) Comparison between the levels of phospholipids (PLs) bound to Mdm12 purified from yeast (red bars) and overall phospholipid levels in yeast (blue bars) showing at least twofold enrichment of PC bound to Mdm12. (Lower) The ESI-MS spectrum reveals the PC species bound to Mdm12. The main PC species is PC(32:2) at m/z 730.5. Lyso-PCs do not bind. (C) In vitro liposome-binding assay to determine the bona fide phospholipid ligands of Mdm12, Mmm1, and their complex. Liposomes prepared with yeast total polar lipid extract (yLL) are incubated with purified His-tagged ERMES proteins (Mdm12, Mmm1Δ5, or their complex). After incubation with liposomes, proteins were purified on nickel-nitrilotriacetic acid (Ni-NTA), and their lipid content was analyzed by HPTLC. The phospholipid contents of proteins incubated (+) or not (−) with liposomes are shown together with a liposome-only control (no protein) showing that no liposome carryover contaminates the protein samples. A phospholipid standard is shown.