Significance
Since the 1980s, many biologists have concluded that the earth is in the midst of a massive biodiversity extinction crisis caused by human activities. Yet fewer than 1,000 of the planet’s 1.9 million known species are officially recorded as extinct. Skeptics have therefore asked “Is there really a crisis?” Mammals and birds provide the most robust data, because the status of almost all has been assessed. Invertebrates constitute over 99% of species diversity, but the status of only a tiny fraction has been assessed, thereby dramatically underestimating overall levels of extinction. Using data on terrestrial invertebrates, this study estimates that we may already have lost 7% of the species on Earth and that the biodiversity crisis is real.
Keywords: biodiversity crisis, invertebrates, IUCN Red List
Abstract
Since the 1980s, many have suggested we are in the midst of a massive extinction crisis, yet only 799 (0.04%) of the 1.9 million known recent species are recorded as extinct, questioning the reality of the crisis. This low figure is due to the fact that the status of very few invertebrates, which represent the bulk of biodiversity, have been evaluated. Here we show, based on extrapolation from a random sample of land snail species via two independent approaches, that we may already have lost 7% (130,000 extinctions) of the species on Earth. However, this loss is masked by the emphasis on terrestrial vertebrates, the target of most conservation actions. Projections of species extinction rates are controversial because invertebrates are essentially excluded from these scenarios. Invertebrates can and must be assessed if we are to obtain a more realistic picture of the sixth extinction crisis.
Status of Invertebrates in the International Union for the Conservation of Nature Red List
Biodiversity decline has been of concern for several decades (1–3). However, the International Union for the Conservation of Nature (IUCN) Red List, the most widely used tool to measure this decline at a global level (4), lists fewer than 800 modern extinctions (5), an infinitesimal fraction of the total number of extant species, commonly estimated at 5–10 million (6, 7). Although the Red List is primarily a tool for identifying those species that are most threatened and thus most in need of conservation action, and not a rigorous catalog of extinctions, reactions to this low number of documented extinctions have ranged from eco-skepticism (8) to eco-satisfaction, the low number seen by some as a measure of the success of conservation programs (9). However, there is a bias in estimates of biodiversity decline, because most of them focus on mammals and birds (10). Assessment of a species’ conservation status according to the IUCN criteria requires robust data on geographic range, population trends, threats, habitat, and ecology, such that the evaluation is rigorous and unassailable. This quality of data is available essentially for only a handful of remarkable species, almost exclusively vertebrates. By 2013, all 15,528 known bird and mammal species had been evaluated against the IUCN Red List criteria (5), with only 5.8% ranked as data deficient (not allocable to one of the other IUCN categories: extinct, extinct in the wild, critically endangered, endangered, vulnerable, near threatened, and least concern). We thus have a fairly good picture of how the biodiversity crisis is impacting mammals and birds, especially large charismatic ones (e.g., rhinos, large cetaceans, tigers, and condors), but we are still in the mist for most invertebrate taxa, a consequence of their poorly documented conservation status (11, 12). Only 15,911 invertebrate species of ∼1.4 million described (13) are listed by IUCN. Of these, only 28% are categorized as data deficient, a proportion that may seem low (i.e., the conservation status of almost three-quarters of the invertebrate species evaluated could indeed be assessed). However, the invertebrate species that have gone through the Red List process belong to relatively charismatic and well-studied groups, such as butterflies, dragonflies, reef-building corals, and certain snails. Because of the lack of information on threats to invertebrates, the vast majority of species are not addressed by the Red List process (14). If, instead, IUCN evaluated randomly chosen invertebrates, the proportion of species that could not be assessed would be much higher. The fact is that after more than four decades of IUCN Red Lists, invertebrates are still essentially unevaluated overall, because for many of these species, the only useful data are, at best, collection dates and localities, sometimes only type localities (13, 15).
Gathering All Available Information on Poorly Known Species
Here, we suggest two alternative methods to assess the conservation status of poorly known species. We test these methods on a sample of land snail species from around the world. Mollusks are particularly suitable for this evaluation, being the group the most impacted by extinction according to the IUCN Red List (16, 17). Moreover, a large community of both professional and amateur scientists has long been involved in recording locality data and vouchering specimens in reference collections. A random worldwide sample of 200 pulmonate land snail species was drawn from the literature and data relevant for evaluating their conservation status were compiled from (i) the literature (SI Discussion), including gray literature, (ii) museum collections, and (iii) consultation with experts (SI Discussion). The literature search resulted in 932 references, of which 80% focused primarily on taxonomy, 32% provided localities, 26% provided dates of collection, and 11% gave information on habitat, range, abundance, and ecology. The collections of five major natural history museums were searched, either via their online databases or directly, to gather additional unpublished data on collection dates and localities. Experts with local knowledge of regional faunas or with global knowledge on particular groups of species were consulted if available. Of the 200 species, nine (4.5%) were already on the Red List (three as extinct, two as threatened, two as near threatened, one as least concern, and one as data deficient). Sixty-one (30.5%) had not been recorded in the field since their original description, and 79 (39.5%) had not been recorded in the previous 50 y (Fig. 1). Sixty-seven species (33.5%) are known from one locality only, and for 37 species (18.5%) we did not find a precise locality (geographic coordinates or named locality that could be pinpointed on a map to within a few kilometers).
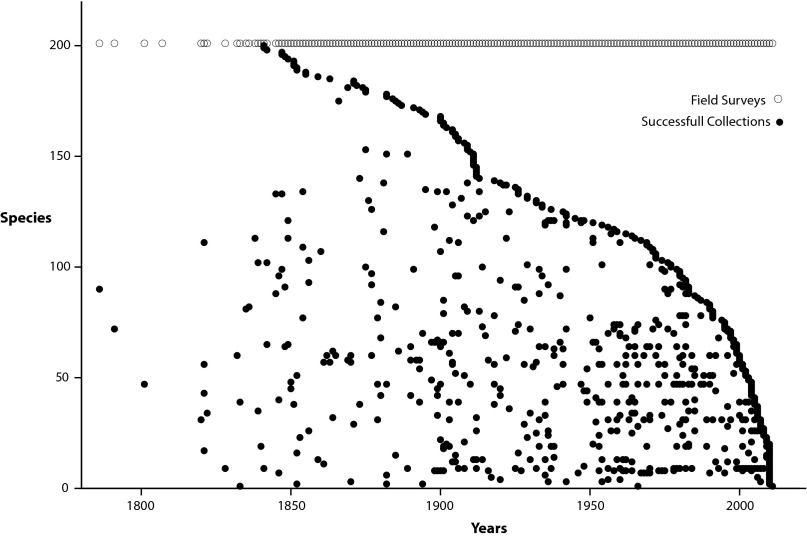
Fig. 1.
Collection dates for 200 randomly selected land snail species. Collection dates, recorded from the literature, museum collections, and consultation with experts for 200 randomly selected land snail species, ordered by date of last collection. Each horizontal line represents all of the collection dates for a single species from 1786 until 2012. Open circles (○; top line) represent all collection dates of mollusks (i.e., proxy for sampling effort). Note the large proportion of species known only from the original collection (e.g., most species between lines 150 and 200).
Results
Experts Assess 10% of the Sampled Species As Extinct.
Data on ecology and distribution, of the robustness required for standard IUCN assessments (SI Discussion), were found for only 31 species; thus, according to the IUCN criteria, 84.5% of our sample would be listed as data deficient in the Red List (Fig. S1). However, based on expert knowledge of species and threat levels, we suggest an alternative assessment method (SI Discussion), and provide the following two examples of the approach. Amastra baldwiniana, endemic to the Hawaiian island of Maui (and member of the endemic Hawaiian family Amastridae), would not be listed as extinct under the IUCN criteria because no targeted surveys have been conducted to search for the species and because no confirmed date of extinction is known. However, it is well understood (16, 18, 19) that amastrids have declined drastically in the Hawaiian Islands through loss of habitat and introduction of invasive species; and, given the taskforce of both professional land snail researchers and amateur shell collectors in Hawaii, and the manageable size of the area to be searched, it is very unlikely that this species would not have been found in the last few decades if it still survived. Consequently, the experts are reasonably sure that this species is extinct, and we would classify it as such. Conversely, Eucalodium moussonianum (Urocoptidae), from Mexico, has not been recorded since its original description in 1872 from the State of Vera Cruz, and there are no data on its distribution or habitat preferences. Given the paucity of field surveys in Mexico, the possibility that the species still survives cannot be excluded, and we would classify it as impossible to assess. Based on this approach, 91 species (45.5%) would be assessed as not threatened, seven (3.5%) as threatened, 20 (10%) as extinct, and 82 (41%) as impossible to assess. Note that these categories are parallel but not identical to the IUCN categories (Materials and Methods).
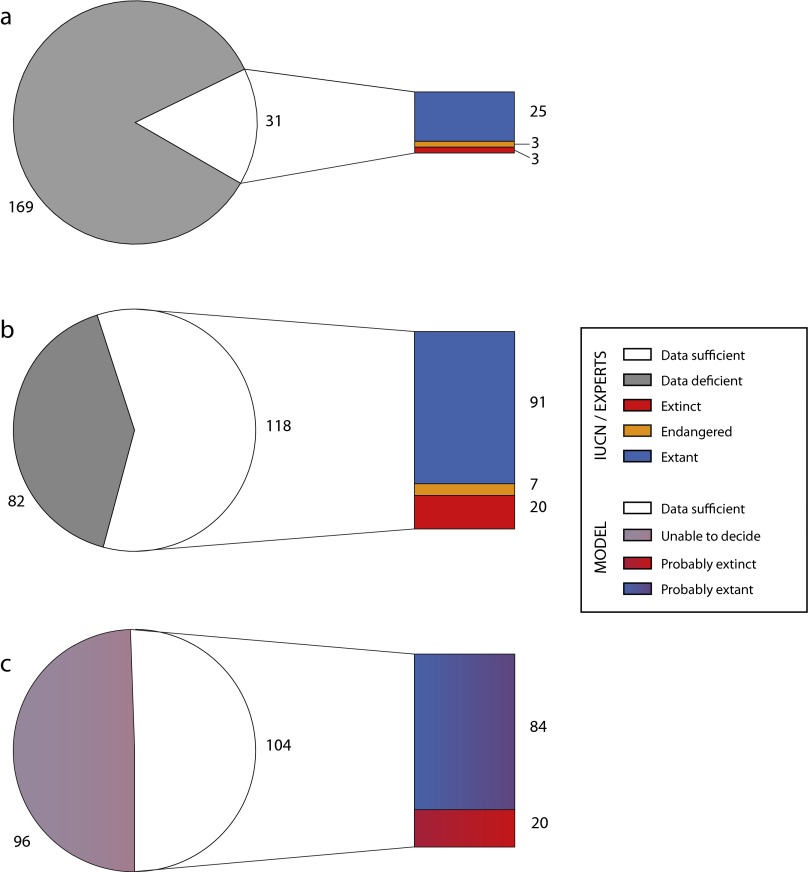
Fig. S1.
Number of mollusk species in our sample assigned to the different extinction risk categories: (A) following IUCN criteria; (B) following our assessment; and (C) following the model prediction. The IUCN categories are simplified in this figure such that endangered includes the categories critically endangered, endangered, and vulnerable, and extant includes the categories least concern and near threatened. Color coding of the model assessments are graded to reflect the probabilistic nature of the assessments.
Stochastic Modeling Approach Is Congruent with Expert Approach.
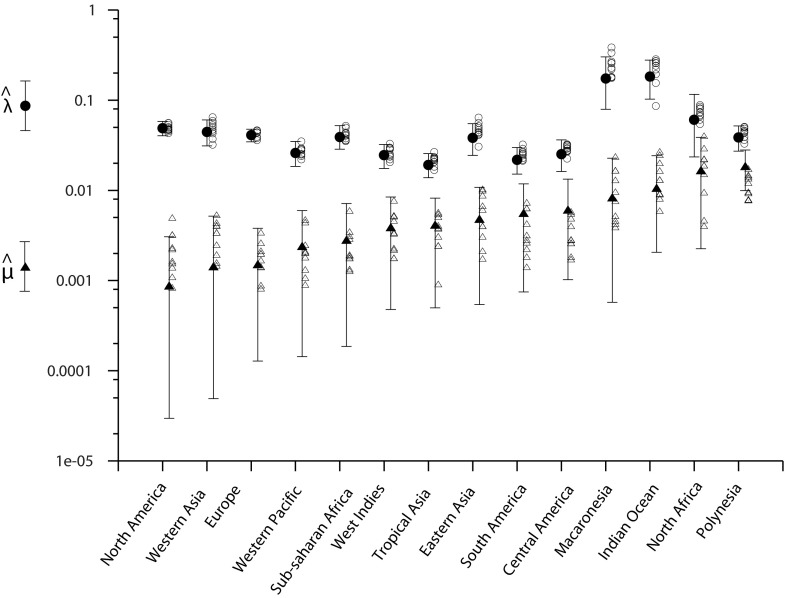
Because the method outlined above relies mostly on supposition derived from interpretation of data and expert opinion and therefore is somewhat subjective, we compared the results with those from a mathematical modeling approach that uses collection records. Similar approaches have been implemented to assess extinction dates of well-known extinct mammal and bird species (20, 21). We inferred an extinction probability for each of our 200 species by comparing dates of collection of a species with overall land snail sampling effort. Dates of sampling of land mollusks were compiled from the entire collections of four major museums (SI Discussion), and the number of collection dates was used as a proxy for sampling effort. The dataset was split into 14 geographic areas (Fig. S2) according to their similarities in terms of biogeography, history of sampling, and factors driving species to extinction. A discrete probabilistic model was built (Materials and Methods and SI Discussion) to estimate three parameters for each species: a global date of extinction rate shift (S), after which extinctions become possible, and, for each geographic area, a probability of extinction per year (µ) and a probability of collecting the species when field work took place (λ) (Fig. 2).
Fig. S2.
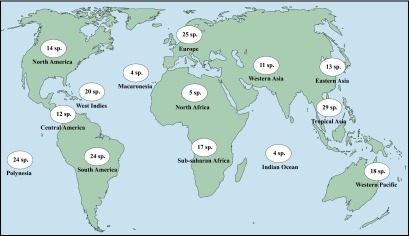
Number of land snail species assessed in the 14 geographic areas recognized.
Fig. 2.
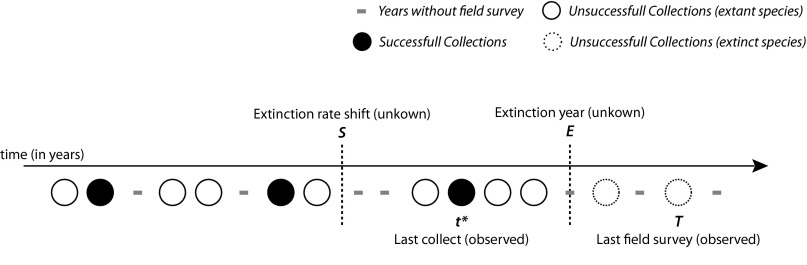
Schematic representation of data used in the probabilistic model for a single hypothetical species. Black circles (●), years in which the species was found; open circles (○), years when searches were undertaken but the species was not found; dashes, years in which there were no searches. The year in which the species was last seen is designated t*, and the year of the last survey is T. Extinction can happen only after year S (year of extinction rate shift), and after the last successful search. The extinction year (E) is unknown. Given the sampling effort before and after the last collection (number of searches, successful or not), the model assesses whether not finding a species is due to extinction or to insufficient sampling.
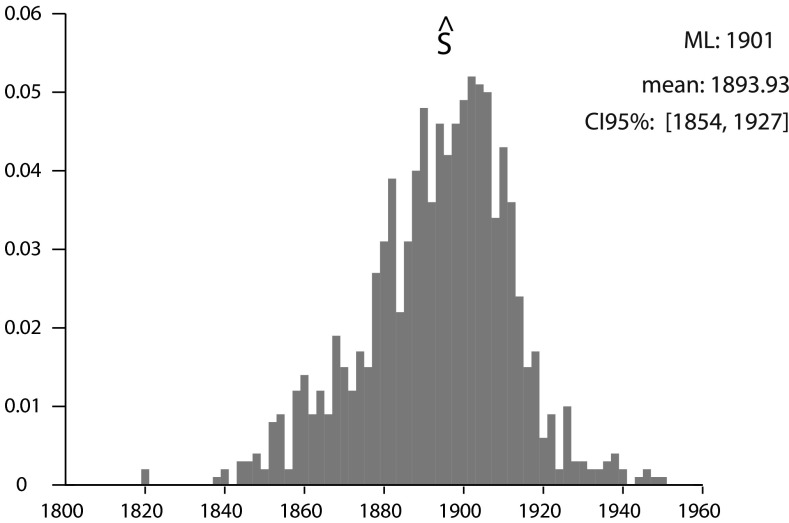
The date S of the extinction rate shift was estimated by pooling all species and finding the mean of its a posteriori distribution. The estimate of S found by this method is 1894 (Fig. S3). The historical context can be invoked to explain this result. The end of the 19th century was the time of the second industrial revolution, that mostly happened in the Western world, but was concomitant with extensive human colonization of preserved natural habitat all over the world, such as in North America or in the midelevation areas of oceanic islands. Because of the preponderant role of recent extinctions in Polynesia in our data, at least the second explanation should be considered seriously.
Fig. S3.
Posterior distribution of S. Using a uniform prior between 1800 and 1950, we retrieved the marginal posterior distribution of S using MCMC on the whole data set. The distribution is clearly unimodal and centered around 1900 and gives an estimate of the date when the extinction rate rose suddenly on a worldwide scale.
The µ and λ parameters were estimated independently for each of the 14 geographic areas. Then, for each species of each geographic area, using the three estimated parameters, we computed the ratio of “the probability of being currently extant but not collected” to “the probability of being extinct” to assess its status.
The expert and model approaches lead to the same general result (Fig. 3; Fig. S1), that the number of extinct species is closer to 7% of biodiversity (130,000 known nonmarine animal species extinctions out of 1.9 million described species) than to the 0.04% currently suggested by the IUCN Red List [if we accept the current figure of 799 extinct species in the Red List (5) and 1.9 million named species (13)].
Fig. 3.
Number of mollusk species in our sample assigned to the different extinction risk categories. Assessments are given according to the model prediction, the expert assessment, and the IUCN criteria. The IUCN categories are simplified in this figure such that endangered includes the categories critically endangered, endangered, and vulnerable, and extant includes the categories least concern and near threatened. Color coding of the model assessments are graded to reflect the probabilistic nature of the assessments.
Fig. 3 shows clear heterogeneity in extinction numbers among geographic areas. In Polynesia, ∼75% of the sampled species are considered extinct by both approaches. In North America, where species have on average much larger ranges than in oceanic islands and are thus less prone to extinction, more than 90% of the sampled species are extant. Large-range species also occur in Africa and in South America, but North America is comparatively more surveyed, hence the lower number of impossible-to-assess species.
There are some discrepancies between the results of the two approaches, but their explanation is straightforward (SI Discussion). Generally, however, the model approach is more pessimistic than the expert one: 25 species should be declared extinct as opposed to 20, because for some species that were assessed as extinct by the model, experts suggested that even with recent surveys in the geographic area considered, lack of data on these species could be due to lack of surveying effort. For instance, E. moussonianum (see above) was evaluated as extinct by the model because there have been unsuccessful surveys in Central America as recently as 2011. However, experts classify it as impossible to assess because, despite these surveys, so much of Mexico and Central America, generally, remains insufficiently explored for mollusks, and recent data are limited regarding species status, range, and availability of suitable habitat, knowledge that is not incorporated by the model.
Discussion
Conservation Status Must Be Assessed for Poorly Known Species.
Both approaches, using available data that cannot be integrated into the current IUCN evaluation methodology, suggest that the status of most land snails (and probably nonmarine invertebrates) can indeed be evaluated; if it exists, the expertise of taxonomists should be used, and natural history museums offer a wealth of data suitable for the evaluation of conservation status. With appropriate models, it is possible to allocate a conservation status to a major proportion of the invertebrate species that currently languish in the shadows out of the conservation spotlights, provided that their biological and ecological characteristics make them suitable for this kind of evaluations. Nonmarine arthropods in general, and most specifically insects, share such characteristics with land snails: size, restricted range, rarity, and specific habitat requirements (22, 23). Moreover, most recorded extinctions involve island endemic species, and range size and level of endemism in marine species are poorly documented (24, 25): for these reasons, our approaches should only be extrapolated to nonmarine fauna.
Some may argue that past and current extinction rate predictions have failed to forecast changes in biodiversity because current figures do not reflect the dramatic losses that conservationists had expected to occur 30 y ago (9). Recently, several studies have attempted to refine such predictions by developing a better understanding of the major drivers of biodiversity changes (26, 27), using process-based models and applying them to groups for which sufficient data exist, i.e., mammals and birds. The current Red List underestimates the actual number of extinct and threatened invertebrate species: there are almost seven times as many extinctions in our sample as would have been listed following the IUCN criteria, and we suggest that discrepancies of this order of magnitude, or greater, given that mollusks are one of the better known invertebrate groups, should be expected for other invertebrate groups. We contend that projections of species extinction rates are controversial not because of methodological challenges but because the “other 99%” of biodiversity, i.e., invertebrates (11), are continually excluded from these scenarios. With an “extant until proven extinct” (28) approach, any poorly studied taxonomic group or geographic region is bound to escape the conservation spotlight. However, a number of studies (17, 29, 30) and our own work suggest that invertebrate extinctions are mostly overlooked: we suggest that we have probably already lost 7% of described living species of the world. On oceanic islands, there is evidence that this percentage is much higher (17, 19, 31).
The Red List is primarily a tool for identifying those species that are most threatened and thus most in need of conservation action, and it works well for charismatic species (mainly terrestrial vertebrates) for which it measures levels of threats and success of conservation measures, especially for conservation-dependent large vertebrates such as the California condor, mountain gorilla, or white rhino. For mammals and birds, the IUCN Red List also documents extinction correctly, and the figure it gives of 1.3% of mammal and bird species being extinct is probably quite accurate, all these taxa having been thoroughly evaluated. But the Red List does a poor job of estimating extinction on a global scale across all taxa and should not be used for this purpose: there are many documented invertebrate extinctions that are not in the Red List (17, 32). Our suggestion that 7% of modern species are extinct is closer to the truth than the 1.3% that is based only on the species evaluated in the Red List.
Data acquisition for invertebrates is far behind that for terrestrial vertebrates, yet the IUCN procedure for categorization requires that the data processing should be based on the same categories and criteria as for terrestrial vertebrates. For the bulk of invertebrates, most of the data are locality and date of collection information currently residing in museum collections and the personal collections of professional and amateur taxonomists. Such data are not readily evaluated against the IUCN criteria, unlike the ecological and demographic data that are widely available for terrestrial vertebrates, which are often the result of studies by numerous scientists on just one or a few species.
However, our aim is not to improve the IUCN Red List approach or to adapt its criteria for invertebrates. Instead, we suggest an alternative approach, aimed at measuring levels of extinction globally (and not at evaluating threats and conservation actions). Our approach accepts some level of uncertainty for individual species, but at a large scale, it aims to provide an estimate of the true level of extinction, something which is neither a priority of the Red List nor something that it is designed to accomplish. The Red List and our approach are not mutually exclusive but represent complementary mechanisms for documenting the biodiversity crisis.
Because the expert and model approaches, which we developed independently, are remarkably consistent in this study, our estimated fraction of extinct species appears to be genuinely robust, and this suggests that the current Red List approach grossly underestimates the extinction crisis for invertebrates. Either we essentially only assess vertebrates, butterflies, and a few snails using the strict IUCN criteria, and accept that we have almost no idea for the rest of biodiversity, or we accept some educated guesses by experts, often based on museum collection data, and/or inferences made from proxy data processed through suitable models, to get closer to the real picture. Model-based analyses are reproducible, quantitative, and can be rigorously evaluated. Because there has been a steady effort to render IUCN criteria stricter, quantified, and objective (4), we believe that adding the model approach to our study constitutes a step forward in the evaluation of the extinction crisis.
Materials and Methods
Species Sampling.
In the absence of a global checklist of the terrestrial mollusks of the world, we used Schileyko’s Treatise on Recent Terrestrial Pulmonate Molluscs (33). We first ordered the genera according to Schileyko’s treatment, in which the number of species in every genus is given, allowing us to generate a list of species numbers from 1 to 17,102. Species and subspecies were treated similarly, because they both represent distinct, recognizable taxa, and because subspecies rank is subject to change depending on authors and species concepts. In the rest of the article, we use the term “species” for terminal taxa. We then generated 200 random numbers between 1 and 17,102. For each genus in which one or more of these numbers fell, we generated a list of all known species (from multiple sources, because Schileyko does not treat all species individually) and ordered them alphabetically, each named species in these genera thereby being given a number. We then selected those 200 species corresponding to the original set of 200 random numbers.
Data Compilation.
Bibliographic search.
The following online databases were queried to accumulate as much published information as possible on the 200 species: Thomson Reuters (formerly ISI) Web of Knowledge (including Zoological Record), Biodiversity Heritage Library, Google Books, Google Scholar supplemented by a search on Google and manual search in the extensive malacological library at the Museum National d’Histoire Naturelle (MNHN) in Paris.
Museum collections.
We augmented the bibliographic data with collection date and locality information from the online collection databases of four US natural history museums: Museum of Comparative Zoology, Harvard University; Field Museum, Chicago; National Museum of Natural History, Washington, DC; Academy of Natural Sciences, Philadelphia. In addition, we manually searched the collections of the MNHN on site.
For the model approach, we also compiled all of the known collection dates globally for all terrestrial mollusks from the four museum collection databases (above).
Consultation with experts.
Experts (taxonomists specialized in a given molluscan family or geographic region; list found in Acknowledgments) were asked whether (i) they had personally collected the species in the field, (ii) they could make an educated guess regarding its conservation status, and (iii) they were aware of recent indications, published or unpublished, of where and when this species had been last seen: e.g., published records, museum or personal collections, or personal field work.
Conservation Status Assessments.
Assessment according to the IUCN categories and criteria.
All 200 species were evaluated according to the IUCN criteria and placed in the appropriate category (34) (Fig. S1). Even if some relevant information—e.g., indicating a range reduction—was available for a species, it was considered as data deficient unless we had the minimum set of information required to place it in one of the other formal IUCN categories. These data included countries of occurrence, maps showing the geographic distribution, rationale for the listing (including any numerical data, inferences or uncertainty that relate to the criteria and their thresholds), current population trends, habitat preferences, major threats, and conservation measures.
Assessment according to the experts.
Expert opinion was available for 54 species. Based on expert field work experience, the species were categorized as not threatened, threatened, extinct, or impossible to assess (if experts estimated that not finding a species was possibly due to insufficient search effort and not to the probable extinction of the species). Note that these categories differ from the IUCN categories.
For species for which no expert opinion was available, our assessment was based on the number of dated records and information on their habitat, as follows: (i) species collected alive at least five times between 1962 and 2012 (i.e., in the previous 50 y) and with no specific habitat threat identified were evaluated as not threatened; (ii) species not collected in the last 50 y but with insufficient information on range size and habitat quality were evaluated as impossible to assess; and (iii) species not collected in the last 50 y and occurring in a highly disturbed or vanished habitat shared by other species already listed by the IUCN as critically endangered or extinct were evaluated as extinct (Fig. S1).
Assessment according to the probabilistic model.
Data compilation.
Collection years were compiled for each species in each geographic area (Fig. S2). We use the term “Polynesia” for the sake of simplicity, although our data are from both Polynesia and Micronesia. In addition, for each geographic area A, all years in which mollusk sampling occurred were listed. These two lists were taken from the databases of the four US museums listed previously. For each species, each survey date t was then tagged by a 1 if the species was collected, or a 0 if otherwise.
Model.
The model is diagrammed for one species in one geographic area in Fig. 2. We define S as the date at which extinction becomes possible. Each species extant at year y ≥ S in geographic area A can independently become extinct between year y to year y + 1 with a constant probability µA. Before time S, the probability of extinction is assumed to be 0. S is therefore the date of the extinction rate shift. We also assume that, at each survey date t, each extant species in the geographic area A, independently, is successfully collected with a constant probability λA. We assume that λA and µA only depend on the geographic area A and that S is identical for all species and all geographic areas. A species that has been observed most recently at date t* is necessarily extant up until t*. In mathematical terms, we have E > t*, where E is the unknown date of extinction of this species. There are two reasons why a species is not observed in a survey subsequent to t*: the species is extant but was not collected (i.e., the survey preceded E) or the species is already extinct (i.e., E preceded the survey). We define T as the date of the last survey (successful or not). We define n1 as the number of survey dates on which the species has been collected, and n0,y as the number of survey dates prior or equal to year y on which the species was not collected; given this, the likelihood of the data for one species is given by
Pooling species within the same geographic area A, the likelihood of the data are the product of the above likelihoods over all species in A (this assumes species are independent replicates).
Statistical inference.
Our goal was to infer a single S for all data together and (λ,µ) for each geographic area. We first estimated jointly Ŝ, , and for all geographic areas pooled (i.e., using worldwide information). Second, we fixed S to Ŝ, and estimated for each geographic area and . To compute Ŝ, we explored parameter space by Markov chain Monte Carlo (MCMC) with all geographic areas pooled. We used a uniform prior for S in [1800, 1950] and a uniform prior in [0, 1] for both λ and µ. We selected as proposal distributions of the Metropolis–Hastings algorithm the uniform distribution in [0, 30] for S and the uniform distribution in the log-scale between 0.1 and 0.0001 for λ and µ. We performed an optimization by maximum likelihood before running the MCMC to focus on the interesting region of parameter space. We discarded the first 100 steps of the chain as burn-in, and sampled 1,000 values every 20 steps. We checked the convergence of the distributions visually. The posterior S distribution showed a clear mode around 1900 and a mean of the distribution of 1893.96 (Fig. S3). Consequently, we set S to 1894 for all further parameter estimation. We were not able to retrieve S for each geographic area independently, presumably because of a lack of signal. For each area, we then ran an independent MCMC to estimate λA and µA.
To characterize the probability of being extant or extinct, we computed, for each species, the likelihood ratio (LR) of the chance it went extinct since it was observed at t* to the chance of being extant but not found since t*. More precisely, LR is defined as
LR is a continuous positive variable, and gives an indication of the current conservation status of the species: the higher it is, the higher is the probability that the species is extinct. However, to assign conservation status, two thresholds were arbitrarily chosen for LR: when LR is above 10 (i.e., there is a 10× greater probability that the species is extinct than that it is extant but not found), we suggest that the species should be declared extinct; when LR is below 0.1 (i.e., there is a 10× greater probability that the species is extant but not found than that it is extinct), we suggest that the species should be declared extant. Between 0.1 and 10, we declare the species impossible to assess.
To check the MCMC algorithm performance, we built 10 random replicates of time series, for each species, keeping the survey dates as they are, but randomly drawing the extinction dates (using a geometric distribution with the estimated µA) and subsequently the successful surveys (using Bernoulli trials of parameter λA before the extinction date). The results show that the MCMC algorithm performs well, estimating values (λA, µA) that are close to those set in the simulations (Fig. S4).
Fig. S4.
Posterior distributions of λ and µ for the 14 geographic areas. Using uniform priors in [0, 1], we retrieved the marginal posterior distributions of λ (success probability of a field survey) and µ (yearly extinction rate after S) using MCMC on data from each geographic area independently. Solid circles and triangles give the mean estimates of λ and µ, respectively, and vertical bars report the associated 95% credibility intervals. Areas were sorted in increasing order of µ estimates. To assess the performance of the MCMC algorithm, we built, for each geographic area, 10 artificial datasets by simulation assuming the estimated λ and µ. Based on the artificial datasets, we computed estimates of λ and µ using the same MCMC algorithm. Estimates from simulated datasets are indicated as open circles and triangles.
SI Discussion
Results by Geographic Area.
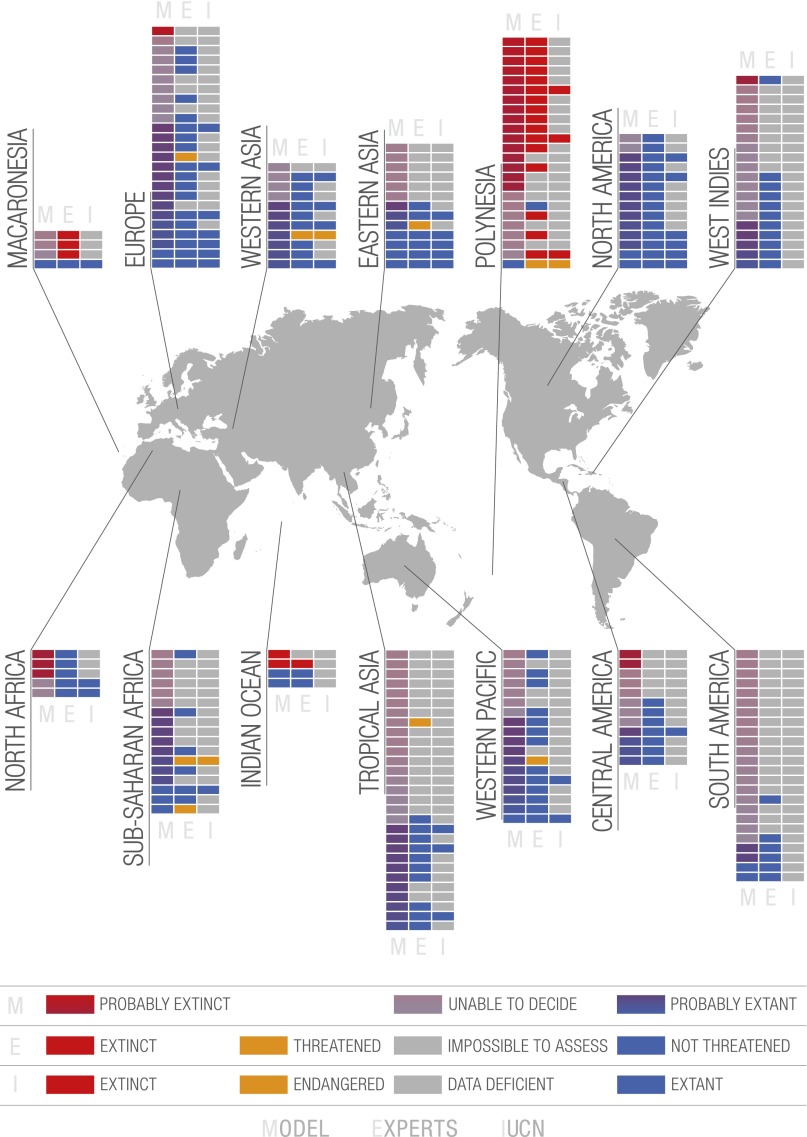
Of the 220 geographic area and species pairs (a few species were collected in more than one geographic area), the bibliographic/collection/experts (henceforth, “expert”) approach and the model approach were quite congruent (Dataset S1); this is particularly the case for Polynesia. Of the 24 species from Polynesia, 16 were evaluated as extinct by the experts and 16 by the model. We have relatively good expertise for this region, and this consistency between the methods reflects the wealth of knowledge we have of these faunas and the mechanisms of their extinction (16, 18, 36–40). We also recorded a high number of extinct Polynesian endemics because these faunas include several species-rich radiations of endemic land snail species and some recent studies have documented extensive recent extinction not only of known biodiversity but also of previously undescribed species (41, 42).
However, species from other areas known for their rich land snail diversity, such as South/Central America and tropical Asia, are also represented in fair numbers in our sample but are not as well known as those from Pacific Islands, most of them having not been studied or even surveyed since their original description in the 19th century. For the 36 and 29 species from South/Central America and tropical Asia, respectively, 23 and 18 species are considered as impossible to assess by the experts, 26 and 18 according to the model. These two regions should be concentrated on in terms of data collection and conservation.
Species from Africa, western and eastern Asia, and the West Indies are less well-represented in our sample, but their biology, habitat preferences, and distribution are as poorly understood as those of species from South America and tropical Asia. As a result, among species listed from these three regions in our sample combined, the experts and model approaches evaluate, respectively, 34% and 39% species as impossible to assess.
These results thus confirm what is already well known: poorly known species occur mostly in developing countries where the biodiversity is the richest and the availability of taxonomists is the poorest (17, 43). Conversely, species for which data availability allows conservation status assessment are either very restricted endemics on islands (i.e., threatened or extinct) or common species with wide ranges, i.e., species that will probably not be threatened in the near future; moreover, they occur in developed countries where experts monitor them: species from North America, Europe, and the western Pacific. For the expert approach we had enough information to evaluate all 14 species (12 for the model) from North America, and they are all considered extant and not threatened. Only 6 of the 25 European species were impossible to assess according to the experts, nine according to the model. For the 18 western Pacific species, only 4 were evaluated as impossible to assess by the experts and 7 by the model. The conservation status of species from Macaronesia and the Mascarene Islands (Indian Ocean) gives cause for concern, especially because they are oceanic island species and therefore intrinsically vulnerable (small ranges, small populations) (44). Among the four Macaronesian species in our sample, three were evaluated as extinct by the experts, and for these same three species the model was unable to decide given the paucity of data for this region. One of the four Mascarenian species was considered extinct by the experts and two by the model.
Discrepancies in Results of Expert and Model Approaches.
For a number of species, the conservation status differed between the expert and the model approaches. Seven types of discrepancies were identified.
Species that experts assessed as threatened.
Our model is designed to infer a probability of extinction, and the status (probably extant/impossible to decide/probably extinct) is then given according to numerical thresholds. Because an endangered species is an extant species, the status inferred by the model for this same species is “alive” because this species was still extant in the field recently. For example, Archachatina machachensis from South Africa was last collected alive in 2010, which is very recent and explains the fact that the probability of extinction computed for this species is null; however, experts noted that its range had shrunk and its habitat had been damaged so they evaluated it as endangered. The taxa concerned are Achatinelloides acutus, Archachatina machachensis, Badistes corneovirens mabillei, Chlamydephorus burnupi, Palaua minor, Parakaliella pagoduloides, and Xerosecta adolfi.
Large-range species.
Some large-range species were attributed to several geographic areas. Because λ and µ were different for each geographic area, a species present in two areas would have different extinction probabilities in each, and may be assessed by the model as extinct in one area and extant in the other. However, experts would assess these species for their whole range: in the part of the range where it is assessed as extinct by the model, it will still appear as assessed as extant by experts. Such species appear twice in the list. Limicolaria turris, for instance, occurs both in Sub-Saharan Africa and in North Africa; it was last collected in 2000 in Sub-Saharan Africa and is considered not threatened. However, the same species was collected once in North Africa in 1863 and therefore is assessed as extinct by the model for this location. The taxa concerned are Coilostele africana, Drymaeus picturata, L. turris, Papillifera bidens affinis, and Trochomorpha swainsoni.
Species living in poorly surveyed areas.
Some species were last collected several decades ago, but experts do not assess them as extinct because no field work has recently been done in their range (even if some has been done in the larger geographic area): they assess them as impossible to assess. However, because the last field sampling of the species was a long time ago, despite more recent field work in the geographic area, the model evaluates such species as extinct; this is the case of Eucalodium moussonianum from Central America, not collected since 1872, and therefore assessed as extinct by the model. Experts know that its range has not been thoroughly surveyed for 60 y and classify this species as impossible to assess. These taxa are Bulimulus inermis, Eostrobilops infrequens, Eucalodium moussonianum, Euplecta acuducta, Irenella pfeifferi, and Lamellidea mumfordi.
Species for which experts disagree.
In a few cases, several experts had different opinions for the same species. In such cases, we had to decide which opinion we would follow, based on habitat preservation in the area, known conservation status of related species, and the validity of the experts’ arguments (e.g., that could be questioned in the light of recent publications). For instance, Lyropupa perlonga from Hawaii was assessed as extinct by two experts who asserted that it had only been collected as a subfossil, and that its habitat is almost completely gone; two other experts assessed it as impossible to assess, considering the paucity of records. Because the last collection date for this species in the field was 1980, the model does not consider it as extinct. In such cases in which experts did not want to decide on the status of a species, we needed to come to a decision. Given our knowledge of the biology of this species in relation to the impact of invasive species, its habitat almost completely gone, the probability of a misidentification of our 1980 record (sustained by two experts), and the fact that this species is already listed as extinct in the IUCN Red List, we evaluated it as extinct. Taxa with similar issues are Lyropupa perlonga and Partulina anceyana.
Species with taxonomic issues.
Taxonomic issues arose concerning two species when they were submitted to experts, although they had never been reported as nonvalid species in the scientific literature; because their taxonomic status is unclear (the biological species designated by their names are unknown), they were classified as impossible to assess. However, due to this taxonomic uncertainty, no data are available apart from their original 19th-century descriptions, and the model evaluates them as extinct. These taxa are Coilostele acus and Perrottetia piriformis.
Species from Macaronesi.
For these species very few collection dates were found (14 dates). In such a situation, it makes no difference for the model if the species was last collected a century ago or 20 y ago. Indeed, the probability of being extant but not collected is ∼0.1 at the lowest. Therefore, there is not enough power to assign a status to these species, and they have been evaluated as impossible to assess, although experts evaluated them as extinct. These taxa are Amphorella leacociana, Actinella crassiuscula, and Madeirovitrina portosantanus.
Species from Polynesia.
Unlike species from Macaronesia, many collection dates are known for species from Polynesia. Two species are only known from the first half of the 20th century (i.e., were not collected in the 19th century and after 1950, despite many collecting events); the probability of successful collection is thus low, and the model evaluates them as impossible to assess, despite their not being found after the 1950s and although experts considered them as extinct. These taxa are Tornatellides attenuatus and Trukrhysa pachystoma dubloni.
Supplementary Material
Acknowledgments
We acknowledge contributions and data sharing from G. M. Barker, A. S. H. Breure, A. C. van Bruggen, C. C. Christensen, D. J. D. Chung, R. Clements, J. Espinosa, A. Fernández Velázquez, J. Gerber, E. Gittenberger, J. Grego, O. Griffiths, M. G. Hadfield, D. Herbert, Y. Kano, W. Maassen, B. Marshall, F. Naggs, E. Neubert, T. A. Pearce, D. Raheem, B. Roth, B. Rowson, R. J. Rundell, M. Severns, J. Stanisic, F. G. Thompson, R. Ueshima, D. Uit de Weerd, A. J. de Winter, and M. Wu. R. Cameron and R. Hershler reviewed an earlier draft of the manuscript. We thank H. Annoni for the design of Fig. 3. We thank C. Fontaine, S. Languille, and H. Le Guyader for comments that improved the manuscript. This work was supported by French National Research Agency Losers Project Grant ANR-09-PEXT-007 (to P.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1502350112/-/DCSupplemental.
References
- 1.Jenkins M. Prospects for biodiversity. Science. 2003;302(5648):1175–1177. doi: 10.1126/science.1088666. [DOI] [PubMed] [Google Scholar]
- 2.Pimm SL, Russell GJ, Gittleman JL, Brooks TM. The future of biodiversity. Science. 1995;269(5222):347–350. doi: 10.1126/science.269.5222.347. [DOI] [PubMed] [Google Scholar]
- 3.Wilson EO. The current state of biological diversity. In: Wilson EO, Peters FM, editors. Biodiversity. National Academy Press; Washington, DC: 1988. pp. 3–18. [Google Scholar]
- 4.Rodrigues ASL, Pilgrim JD, Lamoreux JF, Hoffmann M, Brooks TM. The value of the IUCN Red List for conservation. Trends Ecol Evol. 2006;21(2):71–76. doi: 10.1016/j.tree.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 5. International Union for the Conservation of Nature and Natural Resources (IUCN) (2014) 2014.1 IUCN Red List of Threatened Species (IUCN, Gland, Switzerland)
- 6.May RM. The dimensions of life on Earth. In: Raven PH, Williams T, editors. Nature and Human Society: The Quest for a Sustainable World. National Academy Press; Washington, DC: 2000. pp. 30–45. [Google Scholar]
- 7.Mora C, Tittensor DP, Adl S, Simpson AGB, Worm B. How many species are there on Earth and in the ocean? PLoS Biol. 2011;9(8):e1001127. doi: 10.1371/journal.pbio.1001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lomborg B. The Skeptical Environmentalist: Measuring the Real State of the World. Cambridge University Press; Cambridge, UK: 2001. [Google Scholar]
- 9.Stork NE. Re-assessing current extinction rates. Biodivers Conserv. 2010;19(2):357–371. [Google Scholar]
- 10.Horwitz P, Recher H, Majer J. 1999. Putting invertebrates on the agenda: Political and bureaucratic challenges. The Other 99%: The Conservation and Biodiversity of Invertebrates, eds Ponder WF, Lunney D (Royal Zoological Society of New South Wales, Mosman, New South Wales), pp 398–406.
- 11.Felizola Diniz-Filho JA, De Marco P, Hawkins BA. Defying the curse of ignorance: Perspectives in insect macroecology and conservation biogeography. Insect Conservation and Diversity. 2010;3(3):172–179. [Google Scholar]
- 12.Ponder WF, Lunney D. 1999. The Other 99%: The Conservation and Biodiversity of Invertebrates (Royal Zoological Society of New South Wales, Mosman, New South Wales)
- 13.Chapman AD. 2009. Number of Living Species in Australia and the World. Report for the Australian Biological Resources Study (Department of the Environment, Water, Heritage and the Arts of the Australian Government, Canberra, Australia)
- 14.Collen B, Böhm M, Kemp R, Baillie JEM. Spineless: Status and Trends of the World’s Invertebrates. Zoological Soc London; London: 2012. pp. 1–88. [Google Scholar]
- 15.Stork NE. Measuring global biodiversity and its decline. In: Reaka-Kudla ML, Wilson DE, Wilson EO, editors. Biodiversity II: Understanding and Protecting Our Biological Resources. Joseph Henry; Washington, DC: 1997. pp. 41–48. [Google Scholar]
- 16.Lydeard C, et al. The global decline of nonmarine mollusks. Bioscience. 2004;54(4):321–330. [Google Scholar]
- 17.Régnier C, Fontaine B, Bouchet P. Not knowing, not recording, not listing: Numerous unnoticed mollusk extinctions. Conserv Biol. 2009;23(5):1214–1221. doi: 10.1111/j.1523-1739.2009.01245.x. [DOI] [PubMed] [Google Scholar]
- 18.Cowie RH. Invertebrate invasions on Pacific Islands and the replacement of unique native faunas: A synthesis of the land and freshwater snails. Biol Invasions. 2001;3(2):119–136. [Google Scholar]
- 19.Solem A. 1990. How many Hawaiian land snail species are left? And what we can do for them. Bishop Museum Occasional Papers 30:27–40.
- 20.Elphick CS, Roberts DL, Reed JM. Estimated dates of recent extinctions for North American and Hawaiian birds. Biol Conserv. 2010;143(3):617–624. [Google Scholar]
- 21.Roberts DL, Solow AR. Flightless birds: When did the dodo become extinct? Nature. 2003;426(6964):245. doi: 10.1038/426245a. [DOI] [PubMed] [Google Scholar]
- 22.Basset Y. 1997. Species abundance and body size relationships in insect herbivores associated with New Guinea forest trees, with particular reference to insect host-specificity. Canopy Arthropods, eds Stork NE, et al. (Chapman & Hall, New York), pp 237–264.
- 23.Yeates DK, Bouchard P, Monteith GB. Patterns and levels of endemism in the Australian Wet Tropics rainforest: Evidence from flightless insects. Invertebr Syst. 2002;16(4):605–619. [Google Scholar]
- 24.Hooper JNA, Kennedy JA, Quinn RJ. Biodiversity 'hotspots', patterns of richness and endemism, and taxonomic affinities of tropical Australian sponges (Porifera) Biodivers Conserv. 2002;11(5):851–885. [Google Scholar]
- 25.O'Hara TD. Seamounts: Centres of endemism or species richness for Ophiuroids? Glob Ecol Biogeogr. 2007;16(6):720–732. [Google Scholar]
- 26.Buckley LB, Roughgarden J. Biodiversity conservation: Effects of changes in climate and land use. Nature. 2004;430(6995) doi: 10.1038/nature02717. :2, 33, discussion 33. [DOI] [PubMed] [Google Scholar]
- 27.Pereira HM, et al. Scenarios for global biodiversity in the 21st century. Science. 2010;330(6010):1496–1501. doi: 10.1126/science.1196624. [DOI] [PubMed] [Google Scholar]
- 28.Diamond JM. Extant unless proven extinct? Or, extinct unless proven extant? Conserv Biol. 1987;1(1):77–79. [Google Scholar]
- 29.Dunn RR. Modern insect extinctions, the neglected majority. Conserv Biol. 2005;19(4):1030–1036. [Google Scholar]
- 30.Fonseca CR. The silent mass extinction of insect herbivores in biodiversity hotspots. Conserv Biol. 2009;23(6):1507–1515. doi: 10.1111/j.1523-1739.2009.01327.x. [DOI] [PubMed] [Google Scholar]
- 31.Coote T, Loève E. From 61 species to five: Endemic tree snails of the Society Islands fall prey to an ill-judged biological control programme. Oryx. 2003;37(1):91–96. [Google Scholar]
- 32.Fontaine B, et al. The European union's 2010 target: Putting rare species in focus. Biol Conserv. 2007;139(1-2):167–185. [Google Scholar]
- 33. Schileyko AA (1998–2007) Treatise on recent terrestrial pulmonate molluscs. Ruthenica 1–15(Suppl 2):1–2210.
- 34. International Union for the Conservation of Nature and Natural Resources (IUCN) (2001) IUCN Red List Categories and Criteria: Version 3.1 (IUCN, Gland, Switzerland)
- 35.Cowie RH. Disappearing snails and alien invasions: The biodiversity/conservation interface in the Pacific. Journal of Conchology Special Publication. 2004;3:23–37. [Google Scholar]
- 36.Hadfield MG, Saufler JE. The demographics of destruction: Isolated populations of arboreal snails and sustained predation by rats on the island of Moloka'i 1982–2006. Biol Invasions. 2009;11(7):1595–1609. [Google Scholar]
- 37.Kirch PV, Hunt TL. Historical Ecology in the Pacific Islands: Prehistoric Environmental and Landscape Change. Yale Univ Press; New Haven, CT: 1997. [Google Scholar]
- 38.Richling I, Bouchet P. Extinct even before scientific recognition: A remarkable radiation of helicinid snails (Helicinidae) on the Gambier Islands, French Polynesia. Biodivers Conserv. 2013;22(11):2433–2468. [Google Scholar]
- 39.Sartori AF, Gargominy O, Fontaine B. Anthropogenic extinction of Pacific land snails: A case study of Rurutu, French Polynesia, with description of eight new species of endodontids (Pulmonata) Zootaxa. 2013;3640(3):343–372. doi: 10.11646/zootaxa.3640.3.2. [DOI] [PubMed] [Google Scholar]
- 40.Thibault JC, Cibois A. From early Polynesian settlements to the present: Bird extinctions in the Gambier Islands. Pac Sci. 2012;66(3):271–281. [Google Scholar]
- 41.Abdou A, Bouchet P. Nouveaux gastéropodes Endodontidae et Punctidae (Mollusca, Pulmonata) récemment éteints de l’archipel des Gambiers (Polynésie) Zoosystema. 2000;22(4):689–707. [Google Scholar]
- 42.Preece RC. Impact of early Polynesian occupation on the land snail fauna of Henderson Island, Pitcairn group (South Pacific) Phil Trans R Soc Lond B Biol Sci. 1998;353(1367):347–368. [Google Scholar]
- 43.Gaston KJ, May RM. The taxonomy of taxonomists. Nature. 1992;356(6367):281–282. [Google Scholar]
- 44.Purvis A, Gittleman JL, Cowlishaw G, Mace GM. Predicting extinction risk in declining species. Proc Biol Sci. 2000;267(1456):1947–1952. doi: 10.1098/rspb.2000.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.