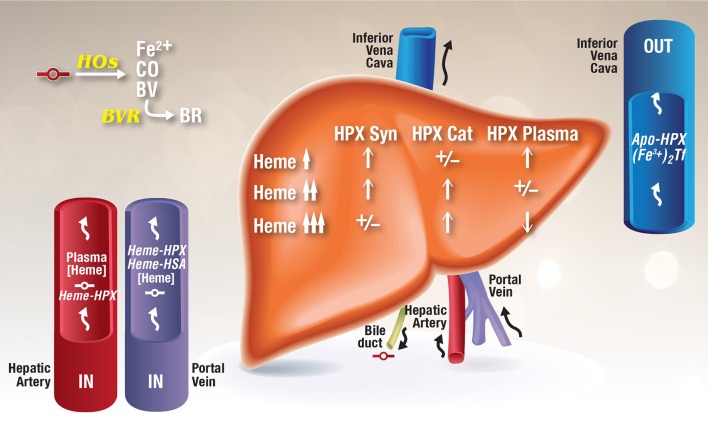
Figure 1.
Model for the development of HPX depletion states. This model is based on data from rhesus monkeys given heme i.v. (Foidart et al., 1982), HPX metabolism studies in humans (Foidart et al., 1983), known induction of HPX by heme (Smith, 1999; He et al., 2010), normal recycling of HPX after heme delivery (Smith and Morgan, 1978, 1979), and HPX catabolism after i.v. administration of heme several fold higher than the binding capacity of HPX (Sears, 1970; Lane et al., 1972). As heme-HPX forms in plasma, uptake of this complex by receptor-mediated endocytosis into liver parenchymal cells raises heme levels. In HPX deficiency states, heme will traffic unregulated into cells. HOs initially degrade this heme releasing redox active ferrous iron, CO, and biliverdin, which is converted to bilirubin by cytosolic biliverdin reductase. Heme also travels to the nucleus to de-repress Bach 1 target genes including HO-1 and also to induce other genes including the HPX gene. As intracellular levels of heme rise in liver cells in response to increases in plasma heme, changes in HPX synthesis and catabolism are reflected in plasma HPX levels as indicated. Heme is a normal component of bile and as hemolysis progresses, unregulated heme diffusion into cells raises heme levels such that any unmetabolized heme can potentially be exported into the bile (Petryka et al., 1977; McCormack et al., 1982).