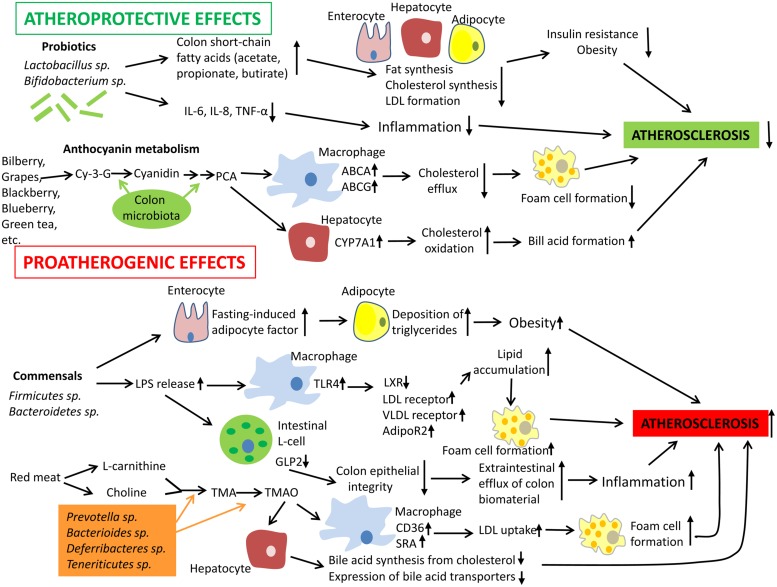
FIGURE 1.
Mechanisms by which gut microbiota could influence the development of atherosclerosis. Intestinal microbiota may have both pro- and anti-atherogenic effects. Probiotics have the atheroprotective activity through the release of bioactive short-chain fatty acids that in turn inhibit fat synthesis in adipocytes and enterocytes and suppress cholesterol biosynthesis and formation of proatherogenic low density lipoproteins (LDL) in the liver. In addition, probiotics and their products could down-regulate inflammation thereby indirectly decreasing the atherosclerosis progression. In addition, anthocyanins presented in some barriers, fruits, and green tea could be metabolized by colon microflora to protocatechuic acid (PCA), a bioactive molecule that could increase expression of ATP-binding cassette transporters ABCA and ABCG and in macrophages and therefore stimulate cholesterol efflux and inhibit macrophage transformation to foam cells, a hallmark of early atherosclerosis. PCA could stimulate cholesterol catabolism and bile acid synthesis via up-regulation of liver cholesterol 7 α-hydroxylase CYP7A1, an enzyme, which oxidizes cholesterol. However, gut microbiota could also exhibit proatherogenic effects. Commensal bacteria such as Firmicutes sp. and Bacteroidetes sp. release lipopolysaccharides (LPS) that could be recognized by Toll-like receptor (TLR)4 and down-regulate expression of transcription factor liver X receptor (LXR)-á (suppressor of cholesterol uptake) while expression of proatherogenic LDL receptor, very low density lipoprotein (VLDL) receptor, and adiponectin receptor 2 (AdipoR2) become up-regulated in macrophages. Indeed, this leads to elevated lipid uptake by macrophages promoting their transformation to foam cells. Furthermore, intestinal-derived LPS were shown to decrease the production of glucagon-like peptide 2 (GLP2) intestinal neuroendocrine L-cells, which results in weakened colon epithelial integrity, increased efflux of colon biomaterial outside the intestine, and enhanced inflammation. Finally, some species of intestinal microbiota were found to show increased capacity to metabolize choline and L-carnitine, which are enriched in red meat, to trimethylamine (TMA) and trimethylamine-N-oxide (TMAO). Elevated levels of TMAO are associated with increased cardiovascular risk. TMAO is able to activate expression of LDL scavenger receptors SRA and CD36 in macrophages thereby stimulating LDL uptake and formation of foam cells. In hepatocytes, TMAO suppress both bile acid synthesis from cholesterol and expression of bile acid transporters. Abbreviations: Cy-3-G, cyanidin-3-O-â-glucoside; IL-6, interleukin 6; TNF-á, tumor necrosis factor á; SRA, scavenger receptor A.