Table 1.
Summary of Study Characteristics (part 1 of 2)
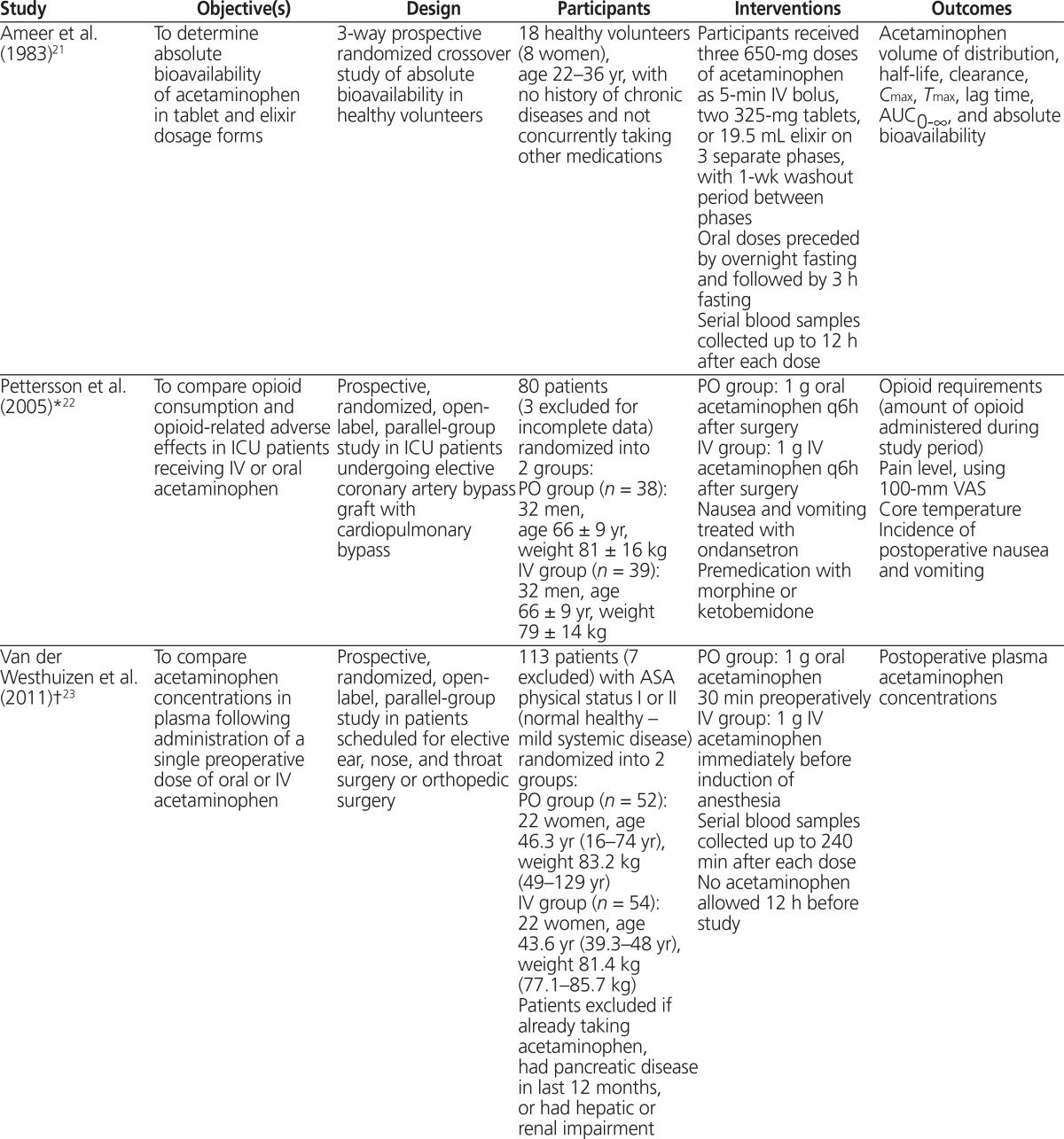
| Study | Objective(s) | Design | Participants | Interventions | Outcomes |
|---|---|---|---|---|---|
| Ameer et al. (1983)21 | To determine absolute bioavailability of acetaminophen in tablet and elixir dosage forms | 3-way prospective randomized crossover study of absolute bioavailability in healthy volunteers | 18 healthy volunteers (8 women), age 22–36 yr, with no history of chronic diseases and not concurrently taking other medications | Participants received three 650-mg doses of acetaminophen as 5-min IV bolus, two 325-mg tablets, or 19.5 mL elixir on 3 separate phases, with 1-wk washout period between phases Oral doses preceded by overnight fasting and followed by 3 h fasting Serial blood samples collected up to 12 h after each dose |
Acetaminophen volume of distribution, half-life, clearance, Cmax, Tmax, lag time, AUC0–∞, and absolute bioavailability |
| Pettersson et al. (2005)*22 | To compare opioid consumption and opioid-related adverse effects in ICU patients receiving IV or oral acetaminophen | Prospective, randomized, open-label, parallel-group study in ICU patients undergoing elective coronary artery bypass graft with cardiopulmonary bypass | 80 patients (3 excluded for incomplete data) randomized into 2 groups: PO group (n = 38): 32 men, age 66 ± 9 yr, weight 81 ± 16 kg IV group (n = 39): 32 men, age 66 ± 9 yr, weight 79 ± 14 kg | PO group: 1 g oral acetaminophen q6h after surgery IV group: 1 g IV acetaminophen q6h after surgery Nausea and vomiting treated with ondansetron Premedication with morphine or ketobemidone | Opioid requirements (amount of opioid administered during study period) Pain level, using 100-mm VAS Core temperature Incidence of postoperative nausea and vomiting |
| Van der Westhuizen et al. (2011)†23 | To compare acetaminophen concentrations in plasma following administration of a single preoperative dose of oral or IV acetaminophen | Prospective, randomized, open-label, parallel-group study in patients scheduled for elective ear, nose, and throat surgery or orthopedic surgery | 113 patients (7 excluded) with ASA physical status I or II (normal healthy – mild systemic disease) randomized into 2 groups: PO group (n = 52): 22 women, age 46.3 yr (16–74 yr), weight 83.2 kg (49–129 yr) IV group (n = 54): 22 women, age 43.6 yr (39.3–48 yr), weight 81.4 kg (77.1–85.7 kg) Patients excluded if already taking acetaminophen, had pancreatic disease in last 12 months, or had hepatic or renal impairment | PO group: 1 g oral acetaminophen 30 min preoperatively IV group: 1 g IV acetaminophen immediately before induction of anesthesia Serial blood samples collected up to 240 min after each dose No acetaminophen allowed 12 h before study |
Postoperative plasma acetaminophen concentrations |
| Brett et al. (2012)‡4 | To compare acetaminophen concentrations in plasma in early postoperative phase following intraoperative IV and preoperative oral acetaminophen | Prospective, randomized, double-blind, parallel-group study in patients scheduled for day-case knee arthroscopy under genera anesthesia | 30 patients with ASA physical status I–II (normal healthy – mild systemic disease) randomized into 2 groups: PO group (n = 20): 8 women, age 50.3 ± 14.7 yr, BMI 27.9 ± 4.1 kg/m2 IV group (n = 10): 3 women, age 50.7 ± 13.3 yr, BMI 27.5 ± 4.4 kg/m2 | PO group: 1 g oral acetaminophen 30–60 min preoperatively IV group: 1 g acetaminophen IV infusion over 15 min Intraoperatively and oral placebo preoperatively to ensure blinding IV fentanyl used in both groups for intraoperative analgesia and as postoperative rescue analgesic if VAS score > 30 mm |
Plasma concentration of acetaminophen 30 min after surgery (primary) Postoperative VAS scores (100-mm scale assessed at 10-min intervals while patient awake in recovery room) Postoperative fentanyl requirements Length of stay in recovery area |
| Singla et al. (2012)25 | To compare plasma and CSF concentration–time curves and PK parameters for acetaminophen after IV, oral, and rectal administration | Prospective, randomized, open-label 3-way crossover study | 7 healthy nonsmoking men, age 19–44 yr, BMI 19–25.6 kg/m2, weight ≥ 50 kg No medications for 7 days before study; no history of excessive bleeding, recent infection, elevated intracranial pressure, neurological disease, or lumbar spine deformities |
Participants received 1-g dose of acetaminophen IV infusion over 15 min, 1-g dose of oral acetaminophen, and 1.3-g dose of acetaminophen rectal suppositories, with 24-h washout period between doses Serial plasma and CSF samples collected up to 6 h after each dose | Acetaminophen Cmax, Tmax, and AUC0–6 in plasma and CSF Plasma half-life of acetaminophen Regular safety assessments and self-reporting of adverse effects |
| Fenlon et al. (2013)26 | To compare postoperative analgesia after preoperative oral and IV acetaminophen | Prospective, randomized, double-blind, double-dummy parallel-group non-inferiority study in patients visiting a maxillofacial outpatient clinic | 128 patients, age 18–65 yr, who underwent at least one lower third molar extraction under general anesthesia randomized into 2 groups: PO group (n = 65): 51 women, age 18.1–57.7 yr, BMI 24.4 ± 4.3 kg/m2 IV group (n = 63): 43 women, age 18.7–54.4 yr, BMI 24.5 ± 5.0 kg/m2 No analgesics or caffeine for 24 h and 6 h before study, respectively | PO group: 1 g oral acetaminophen at least 45 min before surgery IV group: 1 g IV acetaminophen immediately after induction of anesthesia Both groups received appropriate placebo (oral tablets or IV 0.9% normal saline) |
100-mm VAS score at 1 h after surgery Time to request rescue analgesia VAS score at time of rescue analgesia Any adverse events reported |
ASA = American Society of Anesthesiologists, AUC0–∞ = area under the plasma concentration curve, AUC0–6 = area under the plasma concentration–time curve from 0 to 6 h, BMI = body mass index, Cmax = maximum concentration, CSF = cerebrospinal fluid, ICU = intensive care unit, IV = intravenous, PK = pharmacokinetic, PO = oral, q6h = every 6 h, Tmax = time to maximum concentration, VAS = visual analogue scale.
Baseline characteristics presented as mean ± standard deviation.
Baseline characteristics presented as mean (range).
Baseline characteristics presented as mean ± standard error.
