Abstract
Background
Adequately-powered studies directly comparing hard clinical outcomes of darbepoetin alfa (DPO) versus epoetin alfa (EPO) in patients undergoing dialysis are lacking.
Study Design
Observational, registry-based, retrospective cohort study; we mimicked a cluster-randomized trial by comparing mortality and cardiovascular events in US patients initiating hemodialysis in facilities (almost) exclusively using DPO versus EPO.
Setting & Participants
Non-chain US hemodialysis facilities; each facility switching from EPO to DPO (2003–2010) was matched on location, profit status, and facility type with one EPO facility. Patients subsequently initiating hemodialysis in these facilities were assigned their facility-level exposure.
Intervention
DPO versus EPO.
Outcomes
All-cause mortality, cardiovascular mortality; composite of cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke.
Measurements
Unadjusted and adjusted HRs from Cox proportional hazards regression models.
Results
Of 508 dialysis facilities that switched to DPO, 492 were matched with a similar EPO facility; 19,932 (DPO: 9465 [47.5%]; EPO: 10,467 [52.5%]) incident hemodialysis patients were followed up for 21,918 person-years during which 5550 deaths occurred. Almost all baseline characteristics were tightly balanced. The demographics-adjusted mortality HR for DPO (versus EPO) was 1.06 (95% CI, 1.00–1.13) and remained materially unchanged after adjustment for all other baseline characteristics (HR, 1.05; 95% CI, 0.99–1.12). Cardiovascular mortality did not differ between groups (HR, 1.05; 95% CI, 0.94–1.16). Non-fatal outcomes were evaluated among 9455 patients with fee-for-service Medicare: 4542 (48.0%) in DPO and 4913 (52.0%) in EPO facilities. Over 10,427 and 10,335 person-years, 246 strokes and 370 MIs were recorded, respectively. We found no differences in adjusted stroke or MI rates, or their composite with cardiovascular death (HR, 1.10; 95% CI, 0.96–1.25).
Limitations
Non-random treatment assignment, potential residual confounding.
Conclusions
In incident hemodialysis patients, mortality and cardiovascular event rates did not differ among patients treated at facilities predominantly using DPO versus EPO.
Keywords: Mortality, Cardiovascular, Safety, Myocardial Infarction, Stroke, hemodialysis, darbepoetin alfa (DPO), epoetin alfa (EPO), recombinant erythropoietin, erythropoiesis-stimulating agent (ESA), renal replacement therapy (RRT)
Anemia is a common complication of chronic kidney disease (CKD) that affects the vast majority of patients with end-stage renal disease (ESRD) receiving hemodialysis. Treatment with an erythropoiesis-stimulating agent (ESA), often in conjunction with intravenous iron supplementation, constitutes the standard of care for correction of anemia. In April 2013, 85.6% of US hemodialysis patients were estimated to have received ESA treatment.1 In the United States, epoetin alfa (EPO) is the predominant ESA used by large dialysis chains, whereas the more recently approved longer-acting ESA, darbepoetin alfa (DPO), is mainly used in independent and hospital-based dialysis units.2 In 2011, 94.1% of ESA-treated US hemodialysis patients used EPO and 5.9% used DPO.3 By contrast, DPO is much more commonly used in other countries. For example, in 2011 DPO was used by 65.1% of hemodialysis patients in Canada, 55.2% in France, and 48.2% in Japan.3 While the ability of DPO to raise and maintain hemoglobin concentrations is similar to that of EPO,4, 5 sufficiently powered studies on the comparative safety of DPO versus EPO are lacking.
The recent case of another ESA, peginesatide, which was recalled less than a year after approval by the US Food and Drug Administration due to high rates of death and cardiovascular events not detected during its relatively large phase III program,6, 7 casts doubt on the validity of a “class effect” assumption of comparable safety among other ESAs. A recent meta-analysis of randomized trials that assigned patients with CKD to DPO versus EPO found no difference in mortality, but the upper bound of the 95% confidence interval (CI) exceeded a doubling in risk (odds ratio, 1.33; 95% CI, 0.88–2.01).8 A recent network meta-analysis that examined additional (non-death) outcomes, but included fewer trials, concluded that any comparisons among ESAs of cardiovascular outcomes such as MI or stroke were limited by high uncertainty.9 Hence, we sought to compare the safety of DPO and EPO in a large cohort of typical patients with ESRD initiating maintenance hemodialysis. In this study, we exploited the natural experiment that occurs when facilities make a formulary decision to provide one or the other drug to all or nearly all of its patients.
Methods
Study Rationale
Dialysis facilities contract their medications through pre-specified formularies with a facility typically administrating either DPO or EPO, but rarely both. We considered the choice between DPO and EPO as potentially random relative to patient characteristics because such decisions, particularly at the introduction of a new drug, are primarily based on contracts with drug suppliers. Since it is not expected that patients choose a facility based on whether it uses DPO or EPO, we may have the opportunity to exploit these facility level decisions as a natural experiment. Specifically, we used administrative data to mimic a cluster-randomized design, with clustering based on facility by assigning facilities and their incident hemodialysis patients to a treatment arm based on the practice pattern of their facility. We then matched facility pairs for analytic purposes.
Study Population: Patient Selection, Exposure Assignment, and Follow-up
From the US Renal Data System (USRDS), the national registry of persons with ESRD, we identified from billing codes to Medicare all ESA administrations in 2003–2010. We then defined the proportion of ESA administrations that were for DPO versus for EPO in each hemodialysis facility and calendar month. For each facility, we termed a month a DPO facility-month if ≥95% of administered ESAs in that facility and month were DPO; correspondingly, if ≥95% of administrations were EPO, we considered it an EPO facility-month. All other facility-months were categorized as “mixed”. We restricted our study to independent and hospital-based facilities, as it had previously been shown (and confirmed here) that large dialysis chains almost exclusively used EPO.2 Beginning with the approval of DPO by the US Food and Drug Administration for the US market in 2001, we identified all facility-level switches from EPO to DPO. Among the facilities that almost exclusively administered EPO in the same month and year, we randomly selected one facility matched on geographic region (census division), profit status (for-profit vs. not-for-profit), and facility type (free-standing vs. hospital-based) as reported in the USRDS. This algorithm was applied to all observed facility switches from EPO to DPO.
From the first day of the matching month onwards, we identified all patients regardless of their insurance status who initiated hemodialysis in a DPO facility and its matched EPO facility (inception cohort design). If a facility switched back from predominant DPO to predominant EPO use or its matching EPO facility switched to DPO, both matched facilities were no longer eligible to contribute new incident patients to the study. Patients initiating hemodialysis in a DPO facility were assigned DPO and patients initiating hemodialysis in an EPO facility were assigned EPO as their respective exposures, regardless of whether they actually received DPO, EPO, or no ESA. We used this cohort to study mortality outcomes, which are recorded regardless of payor. Patients were censored at end of available data (December 31, 2010), upon switching to peritoneal dialysis, upon receipt of a kidney transplant, when switching to another hemodialysis facility, or when their facility or its match switched to predominant use of the respective other ESA, or was acquired by a large dialysis chain.
For analyses on non-fatal outcomes, we relied on claims-based data. Therefore, we restricted the cohort to patients who survived 90 days after the initiation of dialysis and who had Medicare Parts A+B as their primary payor on that day. In the US, most patients with ESRD are eligible for Medicare benefits after a 90-day waiting period from the date of ESRD incidence certified in the Medical Evidence Report (form CMS-2728). Patients were followed from day 91 after initiation of hemodialysis until censoring for the reasons listed above, as well as death (for nonfatal outcomes), or loss of Medicare Parts A+B coverage.
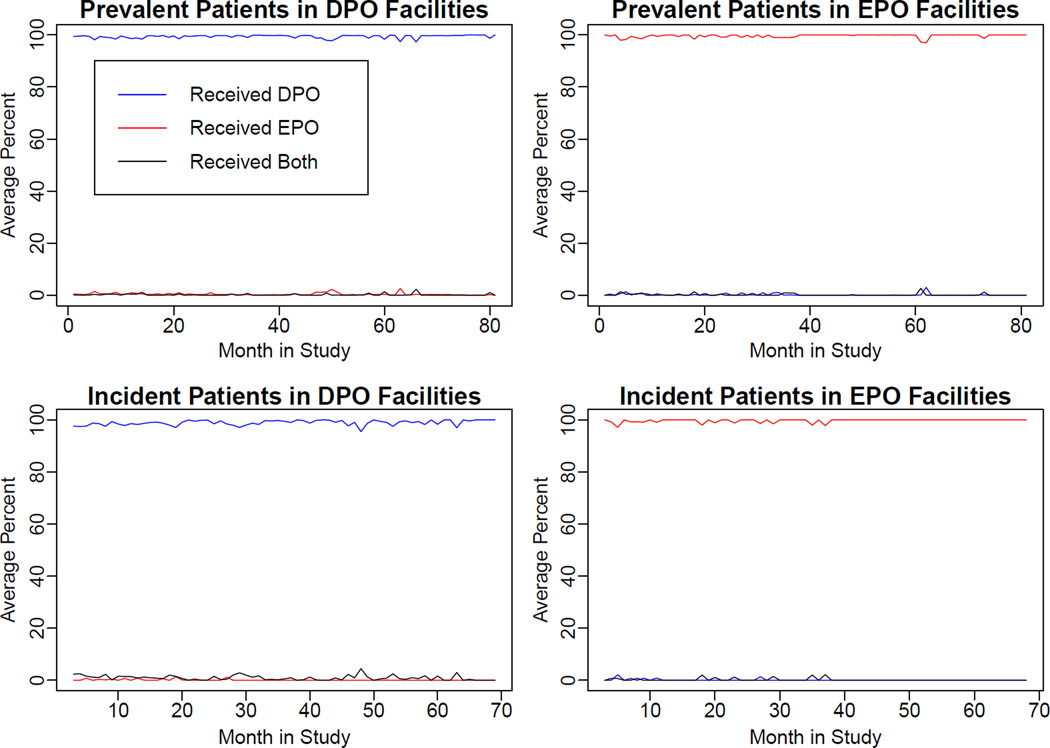
To examine the validity of using facility preference as the proxy for true exposure over time, we plotted for each month of follow-up the percentages of actual ESA received for each exposure group among prevalent and incident patients who had Medicare Parts A+B.
Patient Characteristics
From the USRDS patient file, we ascertained patients’ age, sex, race (white, black, Asian, Native American/Pacific Islander, other), ethnicity (Hispanic vs. non-Hispanic), and whether they were covered by Medicaid (a health insurance program for low-income patients). From the Medical Evidence Report, we ascertained the reported presence of several comorbidities (diabetes, hypertension, arteriosclerotic heart disease, heart failure, peripheral artery disease, cerebrovascular disease, chronic obstructive lung disease, cancer, inability to ambulate or transfer, tobacco use, drug use, alcohol use) as well as body mass index, serum hemoglobin and serum albumin concentrations, and the reported estimated glomerular filtration rate at initiation of dialysis. We also noted whether a patient was reported in the Medical Evidence Report to have received ESAs prior to initiation of dialysis.
Outcomes
Mortality from any cause and cardiovascular mortality were ascertained from the death file in the USRDS, which collates pertinent information from several federal sources. Non-fatal outcomes of interest were ascertained from International Classification of Diseases, 9th Revision, (ICD-9) diagnosis codes (primary diagnosis field) from inpatient Medicare claims using validated algorithms and included stroke (ICD-9: 430, 431, 432.x, 433.x1, 434.x1, 436, 437.1), myocardial infarction (MI; ICD-9: 410.x1), as well as a composite of stroke, MI, and cardiovascular mortality.10
Statistical Analysis
We first tabulated the characteristics of the matched DPO and EPO facilities. We then tabulated the characteristics of all enrolled incident hemodialysis patients by whether they dialyzed in a DPO versus an EPO facility. Groups were compared using standardized difference, with <10% indicating good balance.11 We examined cumulative incidence plots for all outcomes for any differences in event rates or censoring events. We used Cox proportional hazards regression stratified on facility pair to estimate unadjusted hazard ratios (HRs) and corresponding 95% CIs. Schoenfeld residual plots were examined to identify any violations of the proportionality assumption. Since a few characteristics were slightly unbalanced between groups, we also fit demographics-adjusted models and models that included all reported comorbidities and biometric/laboratory characteristics. Missing data were addressed using the MICE (Multivariate Imputation by Chained Equations) package in R statistical software (R Foundation for Statistical Computing).12 We also conducted a set of analyses that were restricted to patients who were reported, per Medical Evidence Report, to have not received any ESA prior to ESRD (ESA naïve). We also inspected cumulative incident plots of all outcomes and censoring events and confirmed in formal competing risk analyses that reported results were unaffected by potential informative censoring (data not shown).
We conducted statistical analyses using SAS software, version 9.3 (SAS Instiute Inc) and R statistical software. The Stanford University School of Medicine Institutional Review Board approved the study.
Results
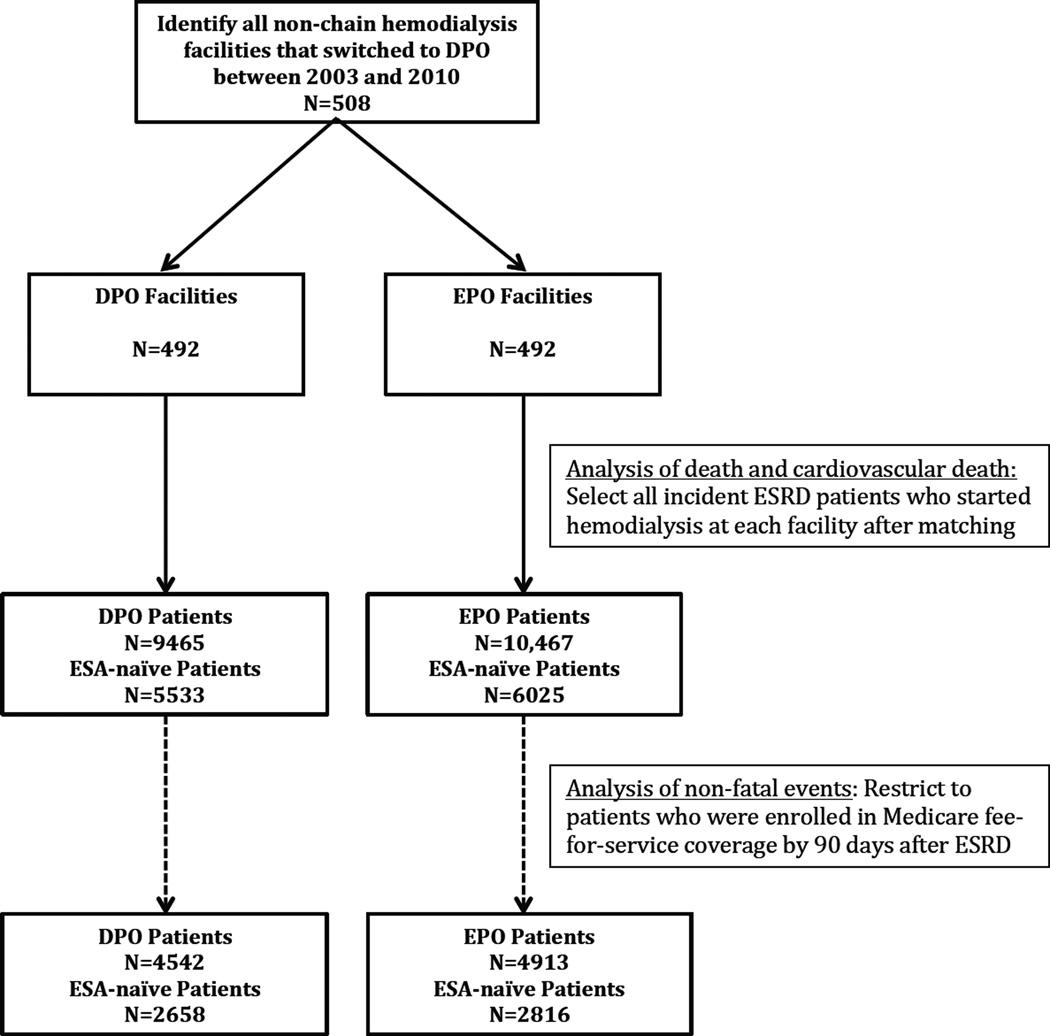
From 2003 through 2010, among 5872 US hemodialysis facilities, 2067 (35.2%) were not part of a chain and of those, 508 (24.6%) facilities switched from EPO to DPO (see Figure S1, available as online supplementary material, for the timing of these switches). Of those, we matched on center type, profit status, and geographic region 492 units (96.9%) with one unit each that had continued to use EPO in the same month and year (Table 1). After the index date and prior to censoring of the matched facility-pair, 19,932 patients initiated hemodialysis in these centers, 9,465 (47.5%) in DPO and 10,467 (52.5%) in EPO facilities (Figure 1), and were followed up for a total of 21,848 person-years. We locked the analytical dataset on October 18, 2013, prior to examining any outcomes and conducted power calculations for fatal and nonfatal endpoints, which demonstrated excellent power for the detection of even small effect sizes (Figure S2). For example, we had 97.8% (53.0%) power to detect a 10% (5%) increase in mortality.
Table 1.
Characteristics of DPO and Matched EPO Facilities
| Variable | DPO (n=492) | EPO (n=492) | ||
|---|---|---|---|---|
| Facility Type | ||||
| Free-standing | 173 (35.2) | 173 (35.2) | ||
| Hospital | 319 | (64.8) | 319 | (64.8) |
| Profit-status | ||||
| Not for profit | 359 | (73.0) | 359 | (73.0) |
| For profit | 133 | (27.0) | 133 | (27.0) |
| Facility size | ||||
| 0–49 patients | 240 | (48.8) | 260 | (52.8) |
| 50–99 patients | 148 | (30.1) | 133 | (27.0) |
| ≥100 patients | 104 | (21.1) | 99 | (20.1) |
| Region | ||||
| Northwest | 102 | (20.7) | 102 | (20.7) |
| Midwest | 215 | (43.7) | 215 | (43.7) |
| South | 106 | (21.5) | 106 | (21.5) |
| West | 69 | (14.0) | 69 | (14.0) |
Note: Values are given as number (percentage). From among 508 facilities that switched from EPO to DPO between 2003 and 2010, we hard-matched one dialysis unit that remained with EPO on facility type (hospital based vs. independent), for-profit status, and geographic region in the month and year of the switching event. We were able to match 492 (96.9%) of facilities that switched to DPO.
DPO, darbepoetin alfa; EPO, epoetin alfa
Figure 1. Numbers of Facilities Matched and Incident Patients Enrolled.
Note: DPO – darbepoetin alfa; EPO – epoetin alfa; ESRD – end-stage renal disease. Whether a patient was ESA naïve was determined from the Medical Evidence Report.
Patient characteristics were similar among patients initiating hemodialysis in DPO vs. EPO facilities with the exception of race and ethnicity (Table 2): DPO facilities had fewer Hispanic and Asian patients, and they also had slightly lower reported serum albumin concentrations. Patient-level separation of ESA exposure during follow-up, assessed from monthly-prevalent patients with Medicare coverage in these units, was excellent as shown in Figure 2. Furthermore, use of intravenous iron and achieved hemoglobin concentrations during follow up were very similar (Figures S3 and S4). Cumulative incidence plots did not indicate any differential censoring between the exposure groups and competing risk analyses yielded essentially identical results (data not shown).
Table 2.
Characteristics of Patients Initiating Dialysis in Matched Darbepoetin Alfa and Epoetin Alfa Facilities
| Variable (% | All Patients | DPO | EPO | Stand Diff |
|||
|---|---|---|---|---|---|---|---|
| No of patients | 19932 (100.0) | 9465 (47.5) | 10467 (52.5) | ||||
| Age (y) | 65 | [53–75] | 65 | [54–76] | 64 | [53–75] | 6.2 |
| Female sex | 8808 | (44.2) | 4159 | (43.9) | 4649 | (44.4) | −1.0 |
| Race* | |||||||
| White | 13515 | (67.8) | 6599 | (69.7) | 6916 | (66.1) | 7.8 |
| Black | 5188 | (26.0) | 2385 | (25.2) | 2803 | (26.8) | −3.6 |
| Asian | 783 | (3.9) | 260 | (2.7) | 523 | (5.0) | −11.7 |
| Other | 422 | (2.1) | 212 | (2.2) | 210 | (2.0) | 1.6 |
| Hispanic ethnicity* | 2410 | (12.1) | 910 | (9.6) | 1500 | (14.3) | −14.7 |
| Medicaid eligibility | 5297 | (26.6) | 2477 | (26.2) | 2820 | (26.9) | −1.9 |
| Received ESA prior to ESRD | 5807 | (29.1) | 2731 | (28.9) | 3076 | (29.4) | −1.6 |
| Comorbidities | |||||||
| Diabetes | 10710 | (53.7) | 5200 | (54.9) | 5510 | (52.6) | 4.5 |
| Hypertension | 16923 | (84.9) | 8157 | (86.2) | 8766 | (83.7) | 6.5 |
| Arteriosclerotic Heart Disease | 5548 | (27.8) | 2627 | (27.8) | 2921 | (27.9) | −0.4 |
| Heart Failure | 7184 | (36.0) | 3453 | (36.5) | 3731 | (35.6) | 1.6 |
| Peripheral Vascular Disease | 3231 | (16.2) | 1558 | (16.5) | 1673 | (16.0) | 1.2 |
| Cerebrovascular Disease | 2238 | (11.2) | 1075 | (11.4) | 1163 | (11.1) | 0.7 |
| Chronic Obstructive Lung Disease | 2273 | (11.4) | 1223 | (12.9) | 1050 | (10.0) | 9.0 |
| Cancer | 1558 | (7.8) | 841 | (8.9) | 717 | (6.9) | 7.5 |
| Unable to ambulate or transfer | 1784 | (9.0) | 861 | (9.1) | 923 | (8.8) | 0.9 |
| Tobacco Use | 1422 | (7.1) | 707 | (7.5) | 715 | (6.8) | 2.4 |
| Drug Use | 422 | (2.1) | 199 | (2.1) | 223 | (2.1) | −0.2 |
| Alcohol Use | 384 | (1.9) | 169 | (1.8) | 215 | (2.1) | −2.0 |
| Reported measurements | |||||||
| BMI (kg/m2)* | 27.3 | [23.4–32.8] | 27.5 | [23.5–33.1] | 27.1 | [23.3–32.5] | 5.7 |
| Hemoglobin (g/dL)* | 9.9 | [8.8–11.0] | 9.9 | [8.9–11.0] | 9.8 | [8.8–10.9] | 6.8 |
| Serum Albumin (g/dL)* | 3.2 | [2.7–3.6] | 3.1 | [2.6–3.6] | 3.2 | [2.7–3.6] | 10.5 |
| eGFR at dialysis initiation (mL/min/ 1.73 m2)* | 9.9 | [7.2–13.3] | 9.7 | [7.0–13.1] | 10.1 | [7.5–13.4] | 8.2 |
Note: Values for categorical variables are given as number (percentage); values for continuous variables, as median [interquartile range].
All incident hemodialysis patients in these facilities were captured for analysis of mortality endpoints regardless of their health insurance status.
Abbreviations: BMI, body mass index; DPO, darbepoetin alfa; EPO, epoetin alfa; eGFR –estimated glomerular filtration rate; ESA – erythropoiesis-stimulating agent; ESRD – end-stage renal disease; Stand Diff – standardized difference.
Percentage missing relevant variable are as follows: race, 0.1%; Hispanic ethnicity, 0.6%; BMI, 1.9%; hemoglobin, 9.1%; albumin, 20.0%; eGFR at dialysis initiation, 2.3%
Figure 2. Actual Treatment Received Among Patients in Darbepoetin Alfa versus Epoetin Alfa Facilities, by Month of Follow-up.
Upper panels show the percentages of prevalent hemodialysis patients with Medicare Parts A+B who received darbepoetin alfa (DPO) versus epoetin alfa (EPO) versus both in each calendar month; Lower panels show the percentages of incident hemodialysis patients with Medicare Parts A+B who received darbepoetin alfa versus epoetin alfa versus both in each calendar month (corresponds to cohort studies for nonfatal outcomes). Left panels are darbepoetin alfa facilities, right panels are epoetin alfa facilities. MPAB – Medicare primary payor, Parts A+B.
During follow-up, 5550 deaths and 2037 cardiovascular deaths occurred for incidence rates of 253.2 and 92.9 per 1000 person-years, respectively (Table 3). Compared with patients who initiated dialysis in EPO facilities, patients in DPO facilities had 12% higher mortality (HR, 1.12; 95% CI, 1.06–1.20), but survival did not differ significantly between groups after adjustment for demographic characteristics (HR, 1.06; 95% CI, 1.00–1.13) or adjustment for all recorded factors (HR, 1.05; 95% CI, 0.99–1.12). Results for cardiovascular mortality were very similar, albeit with wider confidence limits (adjusted HR, 1.05; 95% CI, 0.94–1.16; Table 3).
Table 3.
Follow-up Time, Number of Events, Incidence Rates, and Hazards Ratios; Incident Patients in Hemodialysis Centers Using Darbepoetin Alfa vs. Epoetin Alfa
| Outcomes | Sample Size |
F/U Time (person-y) |
No. of Events |
Incidence Rate (per 1000 person- y) |
Unadjusted | Model 1 | Model 2 |
|---|---|---|---|---|---|---|---|
| Mortality | 19932 | 21917.67 | 5550 | 253.22 | 1.12 (1.06, 1.20) | 1.06 (1.00, 1.13) | 1.05 (0.99, 1.12) |
| Cardiovascular Mortality | 19932 | 21917.67 | 2037 | 92.94 | 1.11 (1.00, 1.24) | 1.05 (0.94, 1.16) | 1.05 (0.94, 1.16) |
| Stroke | 9455 | 10456.87 | 248 | 23.72 | 0.98 (0.72, 1.34) | 1.04 (0.76, 1.43) | 1.02 (0.75, 1.41) |
| Myocardial infarction | 9455 | 10363.29 | 372 | 35.90 | 1.17 (0.91, 1.50) | 1.17 (0.91, 1.50) | 1.16 (0.90, 1.50) |
| Composite^ | 9455 | 10190.52 | 1424 | 139.74 | 1.10 (0.97, 1.25) | 1.09 (0.96, 1.24) | 1.10 (0.96, 1.25) |
Note: Unless otherwise indicated, values are given as hazard ratio (95% confidence interval). Time-to-event analyses started on the day of reported incidence of end-stage renal disease for mortality outcomes and on day 91 after end-stage renal disease for non-fatal and composite outcomes. Model 1 adjusted for age, sex, race, Hispanic ethnicity, Medicaid eligibility, and incidence year. Model 2 additionally adjusted for all comorbidities, body mass index, serum albumin concentration, and estimated glomerular filtration rate.
Multiple imputation was used to address missing data. Results from complete case analyses were not materially different (see Table S2).
Composite of stroke, myocardial infarction, and cardiovascular mortality
For analyses of non-fatal endpoints that were ascertained from medical claims, we identified 9455 incident hemodialysis patients who were alive and covered by Medicare Parts A+B at 90 days after the reported ESRD date; 4542 (48.0%) in DPO and 4913 (52.0%) in EPO facilities. The few imbalances between groups mirrored those of the larger cohort described above (Table S1). Over 10,427 and 10,335 person-years, 246 strokes and 370 MIs were recorded for incidence rates of 23.6 and 35.8 per 1000 person-years, respectively. We found no differences in adjusted stroke or MI rates, or their composite with cardiovascular death (HR, 1.10; 95% CI, 0.96–1.25) (Table 2).
In subgroup analyses of 11,553 patients who were reported to have been ESA-naïve at hemodialysis initiation, results for mortality (HR, 1.01; 95% CI, 0.92–1.10) and cardiovascular mortality (HR, 1.01; 95% CI, 0.87–1.17) were consistent with those obtained from the full cohort (Table S3). Similarly, in the subset of 5474 patients who were ESA naïve, alive and with Medicare coverage at day 90, non-fatal stroke, non-fatal MI, and their composite with cardiovascular mortality were not associated with DPO vs. EPO in adjusted analyses (HR, 1.13; 95% CI, 0.95–1.35).
Discussion
Darbepoetin alfa and EPO are two medications from the ESA class that are commonly used for the treatment of anemia in individuals receiving dialysis and in other settings. While similar in their ability to raise and maintain hemoglobin concentrations in short-term studies,4, 5, 13 longer-term clinical trials that could detect meaningful differences in adverse outcomes are absent. In a meta-analysis of head-to-head trials of DPO versus EPO, no significant difference in mortality was found, but numbers were small, follow-up short, and CIs wide and compatible with sizeable excess mortality in patients randomized to DPO.8 In this study, we leveraged the apparent natural experiment that occurred when facilities switched from EPO to DPO to compare outcomes in patients on hemodialysis. Importantly, we detected no significant differences in death, cardiovascular death, or non-fatal cardiovascular events among patients treated in DPO versus EPO facilities.
We focused on cardiovascular and mortality end-points since previous randomized trials of either DPO or EPO that either compared higher versus lower hemoglobin targets or active ESA versus placebo had identified mostly cardiovascular safety signals. However, the specific safety signals differed across studies, which raises the possibility that the type of adverse outcome depended on the specific ESA used.14 As already stated in our meta-analysis,15 there are well-documented biologic differences between DPO and EPO, with the most important difference for the clinician being the prolonged half-life and increased biological activity for DPO in comparison to EPO.16-19 While the therapeutic aim of ESA treatment is to increase or maintain hemoglobin concentrations in patients with anemia, other effects on additional tissues and organs have been identified, including the brain, heart, uterus, and kidney.20, 21 Erythropoietin may possess pleiotropic properties, stimulating proliferation, chemotaxis, and angiogenesis while downregulating apoptosis.22, 23 It appears to be an important regulator of vascular repair and may also be involved in neoangiogenesis. Both DPO and EPO have been shown to enhance mobilization of bone-marrow derived endothelial-progenitor cells in humans24, 25; these cells play important roles in vascular repair and endothelial regeneration in ischemia-reperfusion injury.21, 26, 27 These non-hematopoietic effects of ESAs, however, may differ from and be disproportional to their relative effectiveness in inducing hematopoiesis. Another derivative of erythropoietin, carbamylated erythropoietin, has been shown to offer similar cardioprotection as epoetin alfa in an animal model, while not exerting an effect on hematocrit.28, 29 This experimental research then raises the important question that we tried to address in this study, namely whether off-target, non-hematopetic, effects may differ between DPO and EPO.
Certain limitations of our study require consideration. Our comparison of DPO and EPO was not randomized and therefore residual confounding remains possible. However, we used an intuitive quasi-experimental approach that mimicked a cluster-randomized trial in which facilities rather than individual patients are randomized to receiving one treatment or another. While the current study was not randomized, treatment with DPO versus EPO was presumably determined by formulary decisions on the facility level, and was found to be mostly independent of patient characteristics. Indeed, after matching facilities by specific criteria such as location, type, and profit status, patients were similar between facilities that had switched to DPO and EPO facilities. Achieved hemoglobin concentrations and use of intravenous iron over the full range of follow-up were very similar between the two groups corroborating that, indeed, the only difference in anemia management was the choice of ESA, but not any presumed or explicit hemoglobin target or iron use strategy. However, we cannot firmly establish from data available to us that facility switches were, indeed, a consequence of facility-wide formulary decisions; alternatively, these switches could have been caused by clinician preferences, nursing workloads, or concerns about EPO safety, and could have been correlated with other facility practices (e.g., dialysis prescriptions, infection control protocols) that may have confounded the estimated associations.
A second limitation comes from the fact that DPO is not widely used in the United States, which required that we restrict our analyses to non-chain freestanding and hospital-based facilities. Whether our results generalize to other healthcare settings such as chain dialysis facilities or to other countries remains unknown. While the ensuing sample size was relatively modest for a national registry analysis, it was more than 20-times the sample size of all randomized trials of DPO vs. EPO combined (and whose endpoints were hemoglobin control), which enabled us to study important outcomes with excellent power. We were also able to demonstrate that there was very little exposure misclassification, with an average 98.9% of ESA recipients in DPO facilities receiving DPO and 99.7% of ESA recipients in EPO facilities receiving EPO throughout follow-up. Thus, statistical power was not diluted by treatment cross-overs. However, not all patients received ESA and not all patients who did received it throughout follow-up, and so a per-protocol analysis of patients treated with ESA would not possess this inherent bias towards the null. Unfortunately, the specific question asked in our study is not addressable by employing causal methods (e.g., marginal structural models) developed to address time-dependent confounding (and our dataset would not have provided the highly-granular data required to support the use of such methods). Our sensitivity analyses of presumably ESA-naïve patients used information provided on the Medical Evidence Report, which has been shown to substantially misclassify pre-ESRD use of ESAs in two validation studies.30, 31 Finally, we were unable to study other ESAs, including epoetin beta, epoetin delta, epoetin omega, biosimilar epoetins, or the long-acting methoxy polyethylene glycol–epoetin beta, all of which are available in other countries, but not currently used in the United States due to patent restrictions.
We conclude that DPO and EPO possess roughly similar safety profiles, at least within the hemodialysis setting, with regard to cardiovascular and mortality outcomes, although small excess risks from DPO cannot be ruled out.
Supplementary Material
Acknowledgements
Support: This work was supported by grant R01 DK090181 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) to Dr Winkelmayer, who also received salary and research support from the endowed Gordon A. Cain Chair in Nephrology at Baylor College of Medicine. Dr Chang (K23 DK095914) and Dr Goldstein (K25 DK097279) were supported by career development grants, and Dr Wilhelm-Leen was supported by institutional training grant T32 DK007357, all from the NIDDK. The manuscript was reviewed for compliance with federal research (privacy) regulations and approved for publication by an officer of the NIDDK. Data reported herein were supplied by the USRDS. Interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Aspects of this work were presented in abstract form at the Eurpoean Renal Assocation–European Dialysis and Transplant Assocation Congress, May 31-June 3, 2014, Amsterdam.
Financial Disclosure: Dr Winkelmayer reports having served within the past 36 months as a scientific advisor to Amgen, Astellas, Astra-Zeneca, Bayer, Fibrogen, GlaxoSmithKline, Keryx, Merck Sharpe & Dohme, Mitsubishi-Tanabe, and Rockwell Pharma, and on data safety monitoring boards for Medgenics and Medtronic. Dr Chertow serves on the Board of Directors of Satellite Healthcare and has served as an advisor to Amgen and Keryx. Dr Brookhart has received investigator-initiated research funding from Amgen and has served as a scientific advisor to Amgen, Merck, Pfizer, and Rockwell Pharma. The other authors declare that they have no other relevant financial interests.
Contributions: Research idea and study design: WCW, MAB, BAG; data acquisition: WCW; statistical analysis: AAM, VD, BAG; supervision or mentorship: WCW, BAG. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. WCW takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Supplementary Material
Table S1: Characteristics of patients initiating dialysis in matched DPO and EPO facilities who had Medicare coverage.
Table S2: Follow-up data for incident patients in DPO vs EPO centers without missing data for variables in model 1.
Table S3: Follow-up data for incident patients in DPO vs EPO centers who were ESA naïve.
Figure S1: Timing of facility switches from EPO to DPO.
Figure S2: Power calculations for study endpoints after dataset locked and before outcomes analyses.
Figure S3: Monthly Hb concentrations among Medicare parts A & B beneficiaries, day 91 onwards.
Figure S4: Monthly proportions of IV iron use among Medicare beneficiaries, day 91 onwards.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
References
- 1.The Dialysis Outcomes and Practice Patterns Study: DOPPS Practice Monitor. Ann Arbor, MI: Arbor Research Collaborative for Health; [accessed 10/26/2013]. Available at http://www.dopps.org/DPM/Files/ESA_use_c_overallTAB.htm. [Google Scholar]
- 2.United States Renal Data System. USRDS 2007 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2007. Chapter 5: Clinical Indicators; pp. 124–136. [Google Scholar]
- 3.2012 Annual Report of the Dialysis Outcomes and Practice Patterns Study: Hemodialysis Data 1997–2011. Ann Arbor, MI: Arbor Research Collaborative for Health; [accessed 10/26/2013]. Available at http://www.dopps.org/annualreport/html/esagroup_c_TAB2011.htm. [Google Scholar]
- 4.Nissenson AR, Swan SK, Lindberg JS, et al. Randomized, controlled trial of darbepoetin alfa for the treatment of anemia in hemodialysis patients. Am J Kidney Dis. 2002;40:110–118. doi: 10.1053/ajkd.2002.33919. [DOI] [PubMed] [Google Scholar]
- 5.Vanrenterghem Y, Barany P, Mann JF, et al. Randomized trial of darbepoetin alfa for treatment of renal anemia at a reduced dose frequency compared with rHuEPO in dialysis patients. Kidney Int. 2002;62:2167–2175. doi: 10.1046/j.1523-1755.2002.00657.x. [DOI] [PubMed] [Google Scholar]
- 6.Fishbane S, Schiller B, Locatelli F, et al. Peginesatide in patients with anemia undergoing hemodialysis. N Engl J Med. 2013;368:307–319. doi: 10.1056/NEJMoa1203165. [DOI] [PubMed] [Google Scholar]
- 7.Macdougall IC, Provenzano R, Sharma A, et al. Peginesatide for anemia in patients with chronic kidney disease not receiving dialysis. N Engl J Med. 2013;368:320–332. doi: 10.1056/NEJMoa1203166. [DOI] [PubMed] [Google Scholar]
- 8.Wilhelm-Leen ER, Winkelmayer WC. Mortality Risk of Darbepoetin Alfa Versus Epoetin Alfa in Patients With CKD: Systematic Review and Meta-analysis. Am J Kidney Dis. 2015 doi: 10.1053/j.ajkd.2014.12.012. 2015 Jan 27. pii: S0272-6386(15)00015-3. doi: 10.1053/j.ajkd.2014.12.012. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmer SC, Saglimbene V, Mavridis D, et al. Erythropoiesis-stimulating agents for anaemia in adults with chronic kidney disease: a network meta-analysis. The Cochrane database of systematic reviews. 2014;12:CD010590. doi: 10.1002/14651858.CD010590.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lenihan CR, Montez-Rath ME, Scandling JD, et al. Outcomes after kidney transplantation of patients previously diagnosed with atrial fibrillation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13:1566–1575. doi: 10.1111/ajt.12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montez-Rath ME, Winkelmayer WC, Desai M. Addressing missing data in clinical studies of kidney diseases. Clin J Am Soc Nephrol. 2014;9:1328–1335. doi: 10.2215/CJN.10141013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Locatelli F, Olivares J, Walker R, et al. Novel erythropoiesis stimulating protein for treatment of anemia in chronic renal insufficiency. Kidney Int. 2001;60:741–747. doi: 10.1046/j.1523-1755.2001.060002741.x. [DOI] [PubMed] [Google Scholar]
- 14.Winkelmayer WC. What caused excess strokes in patients randomized to darbepoetin in the trial to reduce cardiovascular events with Aranesp therapy (TREAT)?: no smoking gun. Circulation. 2011;124:2805–2808. doi: 10.1161/CIRCULATIONAHA.111.071985. [DOI] [PubMed] [Google Scholar]
- 15.Wilhelm-Leen ER, Winkelmayer WC. Mortality Risk of Darbepoetin Alfa versus Epoetin Alfa in Patients with Chronic Kidney Disease: Systematic Review and Meta-Analysis. Am J Kidney Dis. 2015 doi: 10.1053/j.ajkd.2014.12.012. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macdougall IC, Gray SJ, Elston O, et al. Pharmacokinetics of novel erythropoiesis stimulating protein compared with epoetin alfa in dialysis patients. J Am Soc Nephrol. 1999;10:2392–2395. doi: 10.1681/ASN.V10112392. [DOI] [PubMed] [Google Scholar]
- 17.Egrie JC, Browne JK. Development and characterization of novel erythropoiesis stimulating protein (NESP) Nephrol Dial Transplant. 2001;(16 Suppl 3):3–13. [PubMed] [Google Scholar]
- 18.Catlin DH, Breidbach A, Elliott S, et al. Comparison of the isoelectric focusing patterns of darbepoetin alfa, recombinant human erythropoietin, and endogenous erythropoietin from human urine. Clin Chem. 2002;48:2057–2059. [PubMed] [Google Scholar]
- 19.Singh AK. Does TREAT give the boot to ESAs in the treatment of CKD anemia? J Am Soc Nephrol. 2010;21:2–6. doi: 10.1681/ASN.2009111127. [DOI] [PubMed] [Google Scholar]
- 20.Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005;293:90–95. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fliser D, Haller H. Erythropoietin and treatment of non-anemic conditions-- cardiovascular protection. Semin Hematol. 2007;44:212–217. doi: 10.1053/j.seminhematol.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Lappin TR, Maxwell AP, Johnston PG. EPO's alter ego: erythropoietin has multiple actions. Stem Cells. 2002;20:485–492. doi: 10.1634/stemcells.20-6-485. [DOI] [PubMed] [Google Scholar]
- 23.Weiss MJ. New insights into erythropoietin and epoetin alfa: mechanisms of action, target tissues, and clinical applications. Oncologist. 2003;(8 Suppl 3):18–29. doi: 10.1634/theoncologist.8-suppl_3-18. [DOI] [PubMed] [Google Scholar]
- 24.Bahlmann FH, DeGroot K, Duckert T, et al. Endothelial progenitor cell proliferation and differentiation is regulated by erythropoietin. Kidney Int. 2003;64:1648–1652. doi: 10.1046/j.1523-1755.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- 25.Bahlmann FH, De Groot K, Spandau JM, et al. Erythropoietin regulates endothelial progenitor cells. Blood. 2004;103:921–926. doi: 10.1182/blood-2003-04-1284. [DOI] [PubMed] [Google Scholar]
- 26.Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 27.Dzau VJ, Gnecchi M, Pachori AS, et al. Therapeutic potential of endothelial progenitor cells in cardiovascular diseases. Hypertension. 2005;46:7–18. doi: 10.1161/01.HYP.0000168923.92885.f7. [DOI] [PubMed] [Google Scholar]
- 28.Leist M, Ghezzi P, Grasso G, et al. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–242. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- 29.Fiordaliso F, Chimenti S, Staszewsky L, et al. A nonerythropoietic derivative of erythropoietin protects the myocardium from ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2005;102:2046–2051. doi: 10.1073/pnas.0409329102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fischer MJ, Stroupe KT, Hynes DM, et al. Validation of erythropoietin use data on Medicare's End-Stage Renal Disease Medical Evidence Report. Journal of rehabilitation research and development. 2010;47:751–762. doi: 10.1682/jrrd.2009.08.0108. [DOI] [PubMed] [Google Scholar]
- 31.Beaubrun AC, Kanda E, Bond TC, et al. Form CMS-2728 data versus erythropoietin claims data: implications for quality of care studies. Renal failure. 2013;35:320–326. doi: 10.3109/0886022X.2012.747967. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.