Health professionals generally intend to provide effective, safe care and do no harm. Since the Institute of Medicine’s 1999 report To Err is Human brought attention to the overwhelming number of preventable medical errors in hospitals, it has become apparent that numerous threats to patient safety exist. The report estimates that between 44,000 and 98,000 patients die yearly from preventable medical errors. This staggering statistic does not capture patients otherwise harmed, or those who had “near miss” events. Understanding the vulnerabilities of specific patient populations can help providers better protect these patients.
Patients with chronic kidney disease (CKD) are at an inherently increased risk for adverse safety events. Patients with CKD, whether presenting with reduced glomerular filtration rate (GFR) or with kidney damage but preserved function, are at risk of complications from nephrotoxic medications and inappropriate drug dosing. These patients have complications like anemia, hypervolemia, and electrolyte imbalances, along with comorbid conditions like diabetes, hypertension, and heart disease, which prompt frequent health care encounters, thereby increasing patients’ risk for adverse events. Moreover, adverse safety events in CKD have the potential to accelerate loss of kidney function and may increase the risk of end stage renal disease (ESRD) beyond what is expected from the disease’s natural history.
Defining adverse safety events in CKD requires a nomenclature that incorporates various dimensions of safety as they relate to the disease. Box 1 distinguishes between adverse events and safety hazards. The former represent harmful clinical occurrences that are consequences of well-intentioned medical care (as opposed to the natural disease process). Safety hazards include ill-advised practices, omissions, or poorly monitored care that raise the risk of an untoward complication.
Box 1.
Safety nomenclature for CKD care
Adverse Events
|
Safety Hazards
|
Near Miss
|
Abbreviations: CKD, chronic kidney disease; IV, intravenous; ACE, angiotensin-converting enzyme; RAAS, renin-angiotensin-aldosterone system
Compounding its enhanced risk for adverse safety events, CKD is often under-recognized by providers, particularly in elderly patients who may have a seemingly normal serum creatinine but significantly reduced GFR. Delayed recognition (or under-appreciation) of reduced GFR may postpone the initiation of therapies that slow CKD progression, as well as interfere with proper dosing of medications and avoidance of nephrotoxins. The National Kidney Disease Education Program (NKDEP) was launched in 2003 to raise awareness among primary care providers and high risk patients, and advocated for automatic reporting of estimated GFR (eGFR) along with serum creatinine in laboratory reports. Despite this effort, inappropriate medication dosing and nephrotoxic medication prescription among CKD patients persists.
In this Core Curriculum, we review common complications of CKD management and medical interventions that pose significant threats to patient safety. Although dialysis patients and transplant recipients face unique health care dangers, we will focus on those issues specific to non-dialysis-dependent CKD.
DRUG DOSING IN CKD
Improper use and dosing of medications pose a threat to the safety of CKD patients. Many medications used in contemporary healthcare are cleared by the kidneys and require special dosing considerations with reduced kidney function. Studies in a variety of clinical settings show that medications are often inappropriately dosed for GFR and may lead to adverse outcomes. Additionally, adverse drug events that are not dependent on a patient’s kidney function are also more common in CKD. Harmful consequences of such drug-related problems can include acute kidney injury (AKI), other metabolic disturbances, and unexpected or prolonged hospitalization. Polypharmacy and drug interactions may be more common in this population and warrant close attention. Elderly patients are particularly vulnerable to drug dosing errors, as their serum creatinine may not reflect their reduced GFR.
GFR determination prior to medication prescription is advised. This may be accomplished using reliable estimating equations based on serum creatinine (or cystatin C) levels, many of which have been refined in the last decade. The Cockcroft-Gault (CG) formlua has been used as the reference for dosing guidance of many drugs since it was derived in the 1970s, despite its many limitations including its failure to normalize for body surface area and lack of validation against a broad sample of patients with CKD. Newer equations including the Modification of Diet in Renal Disease (MDRD) Study equation and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation are adjusted for body surface area and appear to offer improved accuracy across the range of kidney function. The MDRD Study equation is less accurate than CKD-EPI equation in patients with earlier stages of CKD and in those with type 1 diabetes without proteinuria. Studies comparing dosing recommendations have found significant discordance between those established with the MDRD Study and CG equations. Several studies suggest the CKD-EPI equation may most accurately estimate the GFR for drug dosing. Although earlier drug dosing guidelines were based solely on the CG equation, the US Food and Drug Administration recently endorsed the use of more contemporary means for estimating GFR. Nevertheless, dosing guidance for many drugs remains based on creatinine clearance (CLcr). Notably, all estimations of GFR are most reliable when serum creatinine is in the steady state, and are not validated in the AKI setting where estimation of GFR will overestimate or underestimate kidney function during the evolution of injury and recovery, respectively.
Unfortunately, automated eGFR reporting has had limited success in reducing rates of dosing errors. Providers ultimately must use eGFR in combination with current dosing recommendations, the patient’s clinical status, and benefits versus risks of medications to ensure appropriate dosing. Including prescription dosing decision support in addition to eGFR reporting has demonstrated some reduced rates of drug dosing errors.
Serum drug levels are also affected by factors other than GFR. In CKD, alterations in pharmacokinetic parameters such as absorption, distribution, and metabolism may occur. There is increased volume of distribution of many drugs in patients with CKD due to fluid overload, decreased protein binding, or altered tissue binding. Examples include an increased area under the curve (AUC) after oral administration of sildenafil, and interactions between phosphate binders and certain medications (eg, fluoroquinolones) that decrease their absorption. Since plasma protein binding may be decreased in CKD, it is important to monitor free, unbound drug concentrations for drugs with narrow therapeutic windows, such as phenytoin. Moreover, evidence supports that even drugs with primarily non-renal metabolism can accumulate due to changes in cytochrome P450 activity (eg, the level of CYP3A4 activity has implications for erythromycin metabolism; CYP2C9, for warfarin) in patients with CKD. For these reasons, in addition to GFR, practitioners should monitor patients clinically for evidence of drug toxicity and efficacy, and obtain therapeutic drug measurements when available.
- ». [Accessed January 12, 2015];Frequently Asked Questions About GFR Estimates. 2014 Jan 1; from https://www.kidney.org/sites/default/files/docs/12-10-4004_abe_faqs_aboutgfrrev1b_singleb.pdf. [Google Scholar]
- ».Nyman HA, Dowling TC, Hudson JQ, Peter WLS, Joy MS, Nolin TD. Comparative Evaluation of the Cockcroft-Gault Equation and the Modification of Diet in Renal Disease (MDRD) Study Equation for Drug Dosing: An Opinion of the Nephrology Practice and Research Network of the American College of Clinical Pharmacy. Pharmacotherapy. 2011;31(11):1130–1144. doi: 10.1592/phco.31.11.1130. [DOI] [PubMed] [Google Scholar]
- ».Verbeeck RK, Musuamba FT. Pharmacokinetics and dosage adjustment in patients with renal dysfunction. Eur J Clin Pharmacol. 2009;65(8):757–773. doi: 10.1007/s00228-009-0678-8. [DOI] [PubMed] [Google Scholar]
ANEMIA THERAPY
Anemia is a common complication of CKD owing to insufficient production of erythropoietin in the setting of reduced kidney function. Anemia in ESRD has been associated with increased cardiovascular events, and symptoms of anemia affect quality of life. Therefore, correcting low hemoglobin levels has become common practice in patients with kidney disease. Historically, packed red blood cell transfusions were the treatment of choice. Although transfusion-related infections have become significantly less common due to improved screening methods, other risks including transfusion-related acute lung injury, iron overload, and allosensitization (particularly important in patients who may require organ transplant in the future), do exist.
Erythropoiesis-stimulating agents (ESAs) have been utilized widely to treat CKD-related anemia with the goal of reducing red cell transfusions and potentially protecting against cardiovascular complications. Studies in patients receiving dialysis demonstrate that ESA treatment leads to fewer red blood cell transfusions and improved quality of life. These studies—and observational studies in patients with CKD suggesting that cardiovascular events are reduced with anemia correction—have been extrapolated to support ESA therapy for individuals with CKD. Over the last decade, however, several prospective studies have demonstrated that in patients with non-dialysis-dependent CKD, targeting near-normal hemoglobin levels with ESAs provides no mortality, cardiovascular, or renal benefit. Moreover, several studies have revealed potential harm associated with ESA therapy in CKD. The randomized CHOIR (Correction of Hemoglobin and Outcomes in Renal Insufficiency) trial showed that in patients with nondialysis- dependent CKD, targeting a normal (13.5 g/dL) rather than lower (11.3 g/dL) hemoglobin level resulted in an increased rate of the composite outcome of death, myocardial infarction, hospitalization for congestive heart failure, or stroke, with no difference in quality of life. TREAT (Trial to Reduce Cardiovascular Events with Aranesp Therapy) evaluated ESA therapy for normalization of hemoglobin in patients with CKD, diabetes, and anemia. No difference was seen in either of the primary endpoints of composite time to death/cardiovascular event or time to death/kidney event between the intervention group (given ESA for a target hemoglobin level of 13 g/dL) and the placebo group (given ESA only for rescue therapy for hemoglobin level below 9 g/dL). Additionally, a two-fold increase in stroke and more thromboembolic events were noted, even with post-hoc risk factor adjustment, in the ESA treatment group. ESA trials in CKD have demonstrated evidence that there is an increased risk of mortality associated with ESA use in patients with a history of cancer, and that these agents may contribute to the propagation of certain cancers.
Concerns regarding potential cardiovascular complications from untreated anemia prompt most providers to treat anemia of CKD with ESAs; however, in light of data from the aforementioned trials, hemoglobin targets in the treatment of anemia in CKD remain controversial. The US Food and Drug Administration (FDA) has updated their package insert for ESAs to reflect current evidence that targeting higher levels of hemoglobin carries an increased risk for cardiovascular complications and mortality. The FDA recommends individualized therapy, and does not suggest a specific hemoglobin target. For patients with CKD not on dialysis, FDA advises the consideration of ESA administration only when hemoglobin falls below 10 g/dL, and to reduce the dose or discontinue therapy once the hemoglobin surpasses 10 g/dL. The American Society of Nephrology (ASN), via the Choosing Wisely campaign, also advises against ESA therapy in asymptomatic patients with hemoglobin levels ≥ 10 g/dL.
Iron deficiency should be treated in CKD, and some studies suggest that iron supplementation may delay or reduce the need for ESAs in patients with CKD. Intravenous iron supplementation appears to be better tolerated and more effective than oral preparations. Anaphylactic reactions to intravenous iron can occur with dextran-containing formulations. Otherwise, intravenous iron therapy poses a rare risk of hypersensitivity reaction. Oral iron therapy is complicated by diminished gastrointestinal absorption with food, and interactions with commonly prescribed medications in CKD, including phosphate binders and proton pump inhibitors.
Ultimately, more research is needed to define safe hemoglobin targets for ESA therapy in CKD. Iron deficiency should be treated and practitioners should then weigh the risks and benefits of ESA therapy based on current data and recommendations.
- ».Hazzan AD, Shah HH, Hong S, Sakhiya V, Wanchoo R, Fishbane S. Treatment with erythropoiesis-stimulating agents in chronic kidney disease patients with cancer. Kidney Int. 2014;86(1):34–39. doi: 10.1038/ki.2013.528. [DOI] [PubMed] [Google Scholar]
- ».Macdougall IC, Geisser P. Use of Intravenous Iron Supplementation in Chronic Kidney Disease. IJKD. 2013;7(1):9–22. [PubMed] [Google Scholar]
- ».Pfeffer MA1, Burdmann EA, Chen CY, et al. A Trial of Darbepoetin Alfa in Type 2 Diabetes and Chronic Kidney Disease. N Engl J Med. 2009;361(21):2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- ».Singh AK1, Szczech L, Tang KL, et al. Correction of Anemia with Epoetin Alfa in Chronic Kidney Disease. N Engl J Med. 2006;355(20):2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
POTASSIUM
Hyperkalemia (generally accepted as serum potassium > 5.5 mEq/L) reduces the resting membrane potential of the myocardium and predisposes the patient to cardiac arrhythmias. Patients with CKD are at increased risk for hyperkalemia for multiple reasons, including reduced nephron mass, use of medications that block the renin-angiotensin-aldosterone system (RAAS), and excess dietary potassium intake. As normal potassium filtration and secretion are impaired in CKD, alternate pathways of excretion develop. These pathways involve increased gastrointestinal losses of potassium and enhanced distal tubular potassium secretion, resetting the potassium steady state. This adaptive response offers some protection from hyperkalemia, but hyperkalemia in CKD remains a significant safety concern and management problem.
RAAS inhibitors, including angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), are the cornerstones of treatment for CKD but can also contribute to hyperkalemia. In fact, hyperkalemia may occur in up to 10% of patients with CKD receiving RAAS therapy. Multiple studies have shown that combining ACE inhibitors with ARB therapy or RAAS inhibition with direct renin inhibitors offers no clinical benefit and contributes to harm, including hyperkalemia. The recently ceased NEPHRON-D (The Veterans Affairs Nephropathy in Diabetes) study, which randomized patients with diabetes and CKD into treatment arms with combined ACE inhibitor and ARB versus ARB alone, revealed high rates of hyperkalemia (defined as potassium of ≥ 5.5 mEq/L). The risk of hyperkalemia increases if multiple RAAS inhibitors are used or if the RAAS inhibitor is combined with other hyperkalemia-inducing agents like aldosterone antagonists, non-steroidal anti-inflammatory drugs, beta blockers, or heparin. Therefore, these therapeutic combinations should only be considered when the desired clinical outcome cannot otherwise be achieved and potassium levels can be meticulously monitored.
The clinical relevance of medication-induced potassium elevations remains controversial. Mortality risk from hyperkalemia in the hospitalized population has been shown to increase with severity of hyperkalemia, although this risk may be somewhat attenuated in CKD. Despite the risk of death with hyperkalemia, studies show delays in both inpatient and outpatient follow-up of critical hyperkalemia laboratory results. Until more effective systems-based practice improvements are implemented to alert providers of critical potassium abnormalities, prescribers must be diligent with serum potassium monitoring during initiation and dose adjustments of the aforementioned medications, and in determining whether high potassium values are real or pseudohyperkalemia. Dietary potassium restriction by patients, medication reconciliation, and use of polystyrene or other cationic exchange resins are commonly recommended but require more evidence to demonstrate their true efficacy.
It is also important to note that hypokalemia (serum potassium < 3.5 mEq/L) has been identified as a possible independent risk factor for mortality in CKD. Thiazide and loop diuretics are common medical therapies that may contribute to hypokalemia. Although more research is needed to clarify the relationship between hypokalemia and mortality in CKD patients, practitioners should be wary not only of potassium elevations but also of significant hypokalemia in these patients.
- ».Einhorn LM, Zhan M, Hsu VD, et al. Frequency of hyperkalemia and its significance in CKD. Arch Int Med. 2009;169(12):1156–1162. doi: 10.1001/archinternmed.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ».Espinel E, Joven J, Gil I, et al. Risk of hyperkalemia in patients with moderate chronic kidney disease initiating angiotensin converting enzyme inhibitors or angiotensin receptor blockers: a randomized study. BMC Research Notes. 2013;6:306. doi: 10.1186/1756-0500-6-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ».Fink JC. Chronic kidney disease: the Effect of CKD Therapies on Serum Potassium Levels. Nat Rev Nephrol. 2010;6(11):633–634. doi: 10.1038/nrneph.2010.127. [DOI] [PubMed] [Google Scholar]
- ».Korgaonkhar S, Tilea A, Gillespie BW, et al. Serum potassium and outcomes in CKD: Insights from the RRI-CKD cohort study. Clin J Am Soc Neph. 2010;5(5):762–769. doi: 10.2215/CJN.05850809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ».Weir MR, Rolfe M. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clin J Am Soc Neph. 2010;5(3):531–548. doi: 10.2215/CJN.07821109. [DOI] [PubMed] [Google Scholar]
- ».Weir MR, Bakris GL, Bushinsky DA, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372(3):211–221. doi: 10.1056/NEJMoa1410853. [DOI] [PubMed] [Google Scholar]
GLYCEMIC CONTROL IN CKD
Diabetes is a leading cause of CKD and a commonly associated comorbid condition. Optimal glycemic control in patients with diabetes and CKD is a cornerstone of disease management. Although adequate management of hyperglycemia may delay the progression of microvascular complications (including incidence of albuminuria and kidney disease progression), CKD itself complicates diabetes management. Indeed hyperglycemia and diabetic ketoacidosis raise the risk of AKI; but, without proper attention to GFR and appropriate dose adjustment of diabetic therapy, hypoglycemia may occur. Hypoglycemia has been associated with increased inpatient and outpatient mortality, including in patients with CKD.
While insulin is a staple of diabetes therapy, The Joint Commission on Accreditation of Healthcare Organizations named it one of the top 5 most dangerous medications for all hospitalized patients. The danger of insulin is due to—among other things—improper dose adjustment. Unlike endogenous insulin, which is substantially degraded by the liver, exogenous insulin is primarily eliminated by the kidney. In addition, patients with CKD have reduced peripheral insulin degradation and reduced renal gluconeogenesis. Insulin therapy, therefore, poses an even higher risk to patients with CKD versus those with normal kidney function. Progression of CKD and episodes of AKI with chronic or rapid loss of GFR can lead to accumulation of insulin and subsequent hypoglycemia.
CKD also alters the pharmacokinetics of many oral diabetes medications. Absorption of oral drugs may be altered in the presence of uremic or diabetic gastroparesis, uremia-induced gastritis, volume overload, and by chelating agents such as phosphate binders. Fluctuations in volume status and CKD-related muscle wasting affect the kidney’s ability to regulate the volume of distribution of ingested medications. Impaired excretion of medication also can expose patients with CKD to toxic levels of drugs and their active metabolites, leading to hypoglycemia.
Clearance of metformin, a biguanide and first line glucose-lowering agent, declines in parallel to loss of GFR. Although toxic accumulation of metformin is associated with lactic acidosis, recent evidence shows a strong mortality benefit of metformin use in patients with diabetes and stage 3 CKD. Metformin should be avoided in patients who have CKD along with other conditions that lead to lactic acidosis such as liver failure, hypoxia, and poor perfusion. Cautious use of metformin may otherwise carefully be pursued in early to moderate CKD, with frequent creatinine monitoring and discontinuation for worsening eGFR.
Second generation sulfonylureas, particularly short-acting formulations like glipizide, are generally safe in CKD. Long acting sulfonylureas like glyburide can accumulate in CKD, leading to hypoglycemia; therefore such medications should be avoided in patients with more advanced CKD. Alpha-glucosidase inhibitors such as acarbose and incretin mimetics require adjustment and ultimately discontinuation in late stage CKD and ESRD, respectively. Dipeptidyl peptidase 4 inhibitors may be safely used in CKD, though require dose adjustments as CKD progresses.
In managing patients with both diabetes and CKD, it is critical to balance the benefits of intensive control with the potential harms of aggressive therapy. Ultimately, with close attention to fasting glucose levels and medication adjustments for changing eGFR, hyperglycemia may be safely and effectively managed in the CKD population.
- ».Arnouts P, Bolignano D, Nistor I, et al. Glucose-lowering drugs in patients with chronic kidney disease: a narrative review on pharmacokinetic properties. Nephrol Dial Transplant. 2014;29(7):1284–1300. doi: 10.1093/ndt/gft462. [DOI] [PubMed] [Google Scholar]
- ».Moen MF, Zhan M, Hsu VD, et al. Frequency of hypoglycemia and its significance in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(6):1121–1127. doi: 10.2215/CJN.00800209. [DOI] [PMC free article] [PubMed] [Google Scholar]
PAIN MANAGEMENT IN CKD
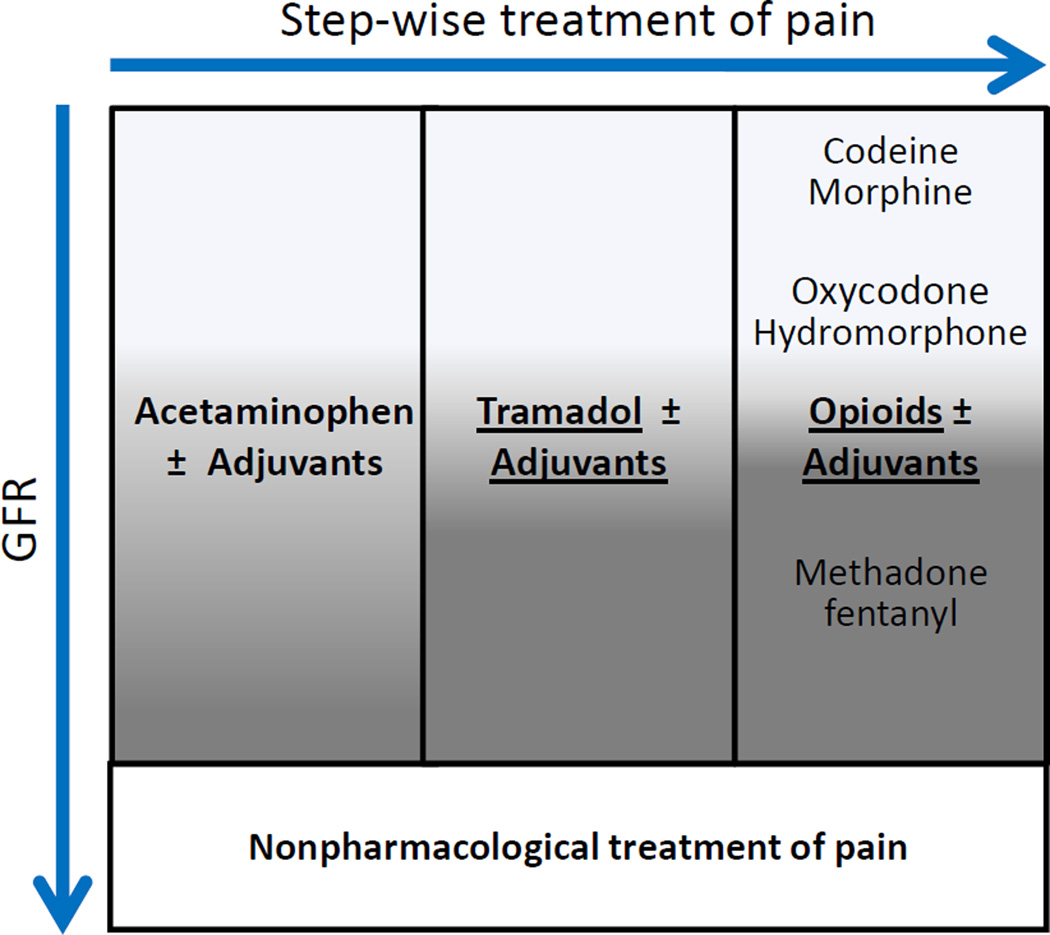
Pain is common in the general population as well as in patients with CKD, and chronic pain has a negative impact on quality of life. A standardized approach to pain management in CKD is challenging because many analgesics have alterations in dosage with reduced kidney function. However, the stepwise therapeutic approach used in healthy patients can still be used in CKD (Figure 1). Acetaminophen is considered the safest non-opioid analgesic, although long-term use and high doses can lead to nephrotoxicity. Nonsteroidal anti-inflammatory drugs (NSAIDs), such as selective COX-2 inhibitors, should generally be avoided in patients with CKD as they not only have direct nephrotoxic effects but also other undesirable side effects. Tramadol remains an acceptable option for moderate pain in patients with CKD. However, decreased elimination of tramadol in advanced CKD can lead to respiratory depression, decreased seizure threshold, and possible risk of serotonin syndrome.
Figure 1.
Analgesic approach in CKD. Caution should be exercised in administering analgesics to patients with reduced GFR. The darker areas indicate situations in which increased caution should be taken (eg, when administering methadone fentanyl to a patient with advanced CKD).
Most opioid analgesics undergo hepatic biotransformation, creating active metabolites that accumulate with reduced kidney clearance. These can lead to respiratory depression, sedation, hypotension, and seizures. Methadone and fentanyl are considered the safest options for severe pain in CKD, while morphine has the most active metabolites cleared by the kidneys and should be avoided. Acceptable adjuvant analgesics include antidepressants (eg, tricyclics, serotonin-norepinephrine reuptake inhibitors), antiepileptics (gabapentin, carbamazepine, pregabalin), muscle relaxants (eg, baclofen), and corticosteroids, but they may require dose and interval adjustment in CKD. Both gabapentin and pregabalin, which are often used for the treatment of diabetic neuropathy, should be dose- and frequency-adjusted depending on degree of CKD. Non-pharmacologic analgesic interventions may be considered to reduce pharmacotherapy for pain in CKD, though little evidence is yet available to demonstrate their efficacy in this context.
Overall, cautious use of narcotic analgesic agents and avoidance of nephrotoxic NSAIDs is recommended for the management of pain in CKD. Close clinical monitoring for evidence of metabolite accumulation is advised.
- ».Phuong-Chi TP, Toscano E, Phuong-Mai TP, et al. Pain management in patients with chronic kidney disease. NDP Plus. 2009;2(2):111–118. doi: 10.1093/ndtplus/sfp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ».Nayak-Rao S. Achieving effective pain relief in patients with chronic kidney disease:a review of analgesics in renal failure. J Nephrol. 2011;24(1):35–40. doi: 10.5301/jn.2010.1330. [DOI] [PubMed] [Google Scholar]
- ».Davison SN, Koncicki H, Brennan F. Pain in chronic kidney disease:a scoping review. Seminars in Dialysis. 2014;27(2):188–204. doi: 10.1111/sdi.12196. [DOI] [PubMed] [Google Scholar]
NEPHROTOXIC MEDICATIONS
Medications are implicated as the etiologic agents of AKI in up to 20% of cases. Hospitalized patients diagnosed with AKI have longer hospitalizations, increased requirements for post-acute care, and increased mortality. Although medication-induced AKI generally appears to be reversible, multiple observational studies show that episodes of AKI can contribute to CKD progression or incident CKD in patients with no history of kidney disease.
Certain patient characteristics are associated with an increased risk of medication-induced AKI, including older age (>65 years), female sex, and comorbid conditions such as diabetes. Reduced GFR from either underlying CKD or prior AKI confers an increased risk for nephrotoxin-induced AKI. Disease states and medications that create absolute or relative hypovolemia also increase the possibility of drug-induced AKI. Examples include gastrointestinal fluid losses, diuretics, sepsis, heart failure, and low protein states such as cirrhosis and nephrotic syndrome. Underlying metabolic conditions that alter urine pH may predispose to intratubular crystal deposition with particular drugs (eg, indinavir). Patients who have electrolyte derangements including hypokalemia, hypomagnesemia, and hypocalcemia are particularly susceptible to nephrotoxicity from aminoglycosides. Risk of AKI is additive with exposure to multiple nephrotoxic agents and increased number of patient-specific risk factors. Providers should review each patient’s clinical risk profile, including the clinical setting and baseline kidney function, prior to the use of nephrotoxins.
Box 2 lists commonly used medications that can cause AKI. Antimicrobial use can lead to various types of AKI, especially in clinical settings involving sepsis-related cytokine release, hypovolemia, and use of vasoactive agents. Most prominent among them are the aminoglycosides, which can produce acute tubular necrosis. Monitoring of serum drug levels may help to minimize toxicity; however, even with vigilant monitoring, drug levels accumulate over time and can cause nephrotoxicity. Gentamicin is considered the most nephrotoxic aminoglycoside followed by tobramycin and amikacin, with streptomycin the least nephrotoxic. Using the least nephrotoxic agent, limiting exposure time to the minimum necessary, and avoiding hypovolemia may reduce the possibility of aminoglycoside-induced kidney damage. Extended-interval dosing is a potential strategy for mitigation of nephrotoxicity. Acute tubular necrosis due to aminoglycoside use is generally considered reversible as proximal tubules can regenerate.
Box 2.
Common Drugs That Cause Nephrotoxicity
| Analgesics |
| Acetaminophen (high doses) |
| NSAIDs |
| Anti-infectives |
| Acyclovir (IV formulation) |
| Aminoglycosides |
| Amphotercin B |
| Beta-lactams |
| Colistin |
| Foscarnet |
| Pentamidine |
| Rifampin |
| Sulfonamides |
| Vancomycin |
| Anti-retrovirals |
| Cobicistat (ARV enhancer) |
| Tenofovir |
| Chemotherapeutics and Immunosuppressants |
| Anti-VEGF agents |
| Cisplatin |
| Cyclophosphamide, Ifosfamide |
| Cyclosporine, sirolimus, tacrolimus |
| Methotrexate |
| Mitomycin-C |
| Other |
| Cocaine |
| Diuretics |
| Iodinated contrast dye |
| Lithium |
| Pamidronate, zolendronate |
| Phenytoin |
| Proton pump inhibitors |
| RAAS blockers |
| Statins |
Abbreviations: ARV, Anti-retroviral; IV, intravenous; NSAIDs, non-steroidal anti-inflammatory drugs; RAAS, renin-angiotensin-aldosterone system; VEGF, vascular endothelial growth factor.
Colistin (polymyxin) is a highly nephrotoxic antimicrobial with a narrow therapeutic window that has resurged recently as an important treatment option for multidrug resistant infections. Recent reviews show that although nephrotoxicity is common with use of colistin, episodes of polymyxin-induced AKI are generally reversible. The anti-fungal amphotericin B is directly toxic to the tubules, particularly in higher doses. Renal toxicity may be attenuated with hydration and is largely reversible with cessation of therapy. The liposomal version of amphotericin B is less nephrotoxic but more costly. Intravenous formulations of the antiviral acyclovir and sulfonamide-based antimicrobials are poorly soluble and may lead to crystal deposition and tubular obstruction. Hydration and alkalinization of the urine with sulfonamide-based agents can reduce risk.
Many drugs have been implicated in causing sporadic cases of acute interstitial nephritis (AIN), but beta lactams and sulfonamide-based drugs are the most widely recognized and common culprits. Onset of AKI within 7–10 days of drug initiation is common with AIN, and hypersensitivity features including rash, fever, eosinophilia, or eosinophiluria may be present. Early recognition and discontinuation of the offending drug improves the likelihood of kidney recovery.
NSAIDs are commonly used medications that confer a high risk of nephrotoxicity through various mechanisms. Most commonly, NSAIDs reduce GFR by decreasing renal prostaglandin synthesis. Patients most susceptible to AKI from NSAID use are those with reduced renal blood flow from hypovolemia, CKD, older age, cirrhosis, heart failure, or concomitant use of other agents (eg, RAAS blockers, diuretics, or iodinated contrast dye). NSAIDs can also induce nephrotic syndrome, papillary necrosis, or AIN. NSAID-induced AIN may present with few if any of the typical hypersensitivity findings generally associated with AIN. Selective COX-2 inhibitors carry a similar risk of nephrotoxicity as their non-selective counterparts. Over-the-counter availability results in widespread NSAID use without physician oversight. Providers can reduce the threat of AKI by counseling patients with high-risk comorbid conditions, polypharmacy, and advanced age to avoid NSAID use. When NSAIDs are indicated in otherwise healthy patients, providers can ensure adequate hydration, prescribe the lowest effective dose for the shortest duration, and avoid using NSAIDs that carry the highest risk of nephrotoxicity (ketorolac and indomethacin). As part of the Choosing Wisely campaign, ASN advises against NSAID use in CKD of all causes.
ACE inhibitors and ARBs decrease intraglomerular pressure by inhibiting angiotensin II–mediated vasoconstriction at the efferent arteriole. This mechanism may result in an increase in serum creatinine and reduced GFR. A rise in serum creatinine of up to 30% is expected and tolerated as long as this change is stable over time and does not lead to other complications of decreased GFR including volume overload, hyperkalemia, or uremia. RAAS-blocker induced AKI may occur when patients have decreased kidney perfusion from hypovolemia, CKD, or bilateral renal artery stenosis. Serum creatinine should be monitored for up to 8 weeks after the initiation of these drugs.
Providers must be vigilant when prescribing nephrotoxic agents in this era of an increasingly vulnerable, aging population with multiple comorbid conditions and polypharmacy. Carefully evaluating patients for inherent characteristics that increase their risk of AKI and judiciously prescribing those drugs that are most nephrotoxic are important steps practitioners can take to protect their patients.
- ».Pannu N, Nadim MK. An Overview of Drug-Induced Acute Kidney Injury. Crit Care Med. 2008;36(4 Suppl):S216–S223. doi: 10.1097/CCM.0b013e318168e375. [DOI] [PubMed] [Google Scholar]
- ».Perazella MA. Renal Vulnerability to Drug Toxicity. Clin J Am Soc Nephrol. 2009;4(7):1275–1283. doi: 10.2215/CJN.02050309. [DOI] [PubMed] [Google Scholar]
ANTIMICROBIALS IN CKD
Patients with CKD may be at increased risk of acquiring infections due to their comorbid conditions and use of healthcare facilities. Some antimicrobials are nephrotoxic and many others are eliminated by the kidneys, leading to adverse effects if not dose-adjusted for reduced kidney function. The majority of beta-lactams (eg, penicillins, cephalosporins, carbapenems) with the exception of a few (eg, nafcillin, ceftriaxone) require adjusting the dose and/or frequency to avoid neurotoxicities such as seizures. Moxifloxacin is the only fluroquinolone that does not require adjustment in CKD as it achieves poor urinary concentrations. Nitrofurantoin should be avoided in patients with CrCl < 50 mL/min as there is insufficient accumulation of the drug to be effective. Sulfamethoxazole-trimethoprim dosing should be reduced in CKD patients. It should be noted that the trimethoprim component of this combination antibiotic can block the proximal secretion of creatinine, thus falsely increasing serum creatinine without changing GFR, and independently prevent potassium excretion in the collecting tubule, leading to hyperkalemia. Aminoglycosides and vancomycin require modifications in dose and dosing intervals, and can cause nephrotoxicity especially with high trough blood levels. Close therapeutic drug monitoring and pharmacokinetic calculations should be used to optimize efficacy while decreasing toxicities. Daptomycin requires a change in dosing interval in patients with CrCl < 30 mL/min. Several antivirals require a decrease in dose and/or frequency (eg, acyclovir, foscarnet). Of the antifungals, fluconazole is most notable for required adjustment in patients with CKD. Providers should utilize drug information sources prior to the prescription of antimicrobials in CKD and adjust the dose and/or frequency as indicated. Although long term antibiotic courses are indicated at times, ASN recommends limited use of peripherally inserted central catheters in late stage CKD so as to preserve veins for arteriovenous fistula placement.
- ».Munar MY, Singh H. Drug dosing adjustments in patients with chronic kidney disease. Am Fam Physician. 2007;75(10):1487–1496. [PubMed] [Google Scholar]
- ».Zuber K, Liles AM, Davis J. Medication dosing in patients with chronic kidney disease. JAAPA. 2013;26(10):19–25. doi: 10.1097/01.JAA.0000435257.26357.a8. [DOI] [PubMed] [Google Scholar]
ANTIPLATELET AND ANTICOAGULANT THERAPY IN CKD
Patients with CKD often require antiplatelet or anticoagulant therapy but have an increased risk of bleeding. Disruptions in the coagulation cascade that occur in CKD include increased fibrinogen, d-dimer, and prothrombin fragments. Pro-coagulant functions of factors XII, XI, IX, VIII, VII, X, and II are enhanced while anticoagulant activities of protein C, protein S, anti-thrombin III, plasminogen, and tissue type plasminogen activator are diminished. Defects in platelet activation, recruitment, adhesion, and aggregation occur with accumulating uremic toxins resulting in dysfunctional platelets.
Rates of acute coronary syndrome (ACS), venous thromboembolism (VTE), and atrial fibrillation (AF) are higher in CKD than the general population. Use of antithrombotic and anti-platelet agents for these conditions in CKD has not been adequately studied because these patients are underrepresented in clinical trials. Thorough bleeding risk assessment should precede antithrombotic use in CKD. Tools including the CHA2DS2-VASc score for stroke risk and HAS-BLED for bleeding risk evaluation in patients with AF can aid in weighing the treatment benefits and risks. In patients with ACS, considerations include timing and type of coronary stent placement and the need for dual versus triple therapy in patients with concomitant AF.
Antiplatelet Agents
Aspirin is primarily metabolized by the liver and is excreted in the urine. Aspirin inhibits the synthesis of renal prostaglandins and their favorable renal hemodynamic effects, increasing the potential for reduced kidney function in CKD patients when used at higher doses. However, established data shows aspirin decreases morbidity and mortality in patients with ACS and coronary artery disease in the CKD population. Therefore, low dose aspirin (<100mg) is recommended in CKD for secondary prevention of cardiovascular disease.
Clopidogrel and prasugrel are oral irreversible inhibitors and ticagrelor is an oral reversible inhibitor of the P2Y12 receptor, a crucial factor in the signaling pathway of platelet activation. While data show no significant difference in outcomes with use versus nonuse of clopidogrel in mild to moderate CKD, data among patients with stage 3 to 5 CKD treated with clopidogrel are consistent with lack of efficacy and increased bleeding. When compared to clopidogrel, prasugrel lead to a lower incidence of ischemic events in patients with normal kidney function or stage 1 to 2 CKD with no differences in patients with stage 3 to 4 CKD. Ticagrelor, when compared to clopidogrel, had a greater reduction in the composite end point of cardiovascular death, myocardial infarction, and stroke in patients with stage 3 to 4 CKD compared to patients with normal kidney function or stage 1 to 2 CKD without increased risk of bleeding. Cautious use of these agents in CKD is recommended with no specific dose adjustments suggested. Through unknown mechanisms, creatinine levels may increase with ticagrelor use specifically in the elderly, patients with late stage CKD, and those receiving ARBs. Thus, ticagrelor should be monitored after its initiation.
Glycoprotein IIb/IIIa inhibitors including abciximab, tirofiban, and eptifibatide block the final step of platelet aggregation and are used in ACS treatment. Abciximab has similar clinical outcomes among patients with and without CKD but poses a possible increase in bleeding in patients with the disease. Abciximab, eliminated via the reticuloendothelial system, requires no dose adjustments in CKD. Tirofiban and eptifibatide require dose adjustments in CKD, and tirofiban specifically has been shown to have increased ischemic and bleeding risk with worsening kidney function.
Anticoagulant Agents
Unfractionated heparin and low-molecular weight heparins such as enoxaparin and dalteparin inhibit Factor Xa and Factor IIa. UFH, primarily metabolized in the liver and endothelium, does not require dose adjustment with decreased kidney function. Low-molecular-weight heparins, however, are predominantly eliminated by the kidneys with decreased clearance in CKD. Their dose should be adjusted for CrCl < 30 mL/min to avoid substantially increased bleeding risk. Enoxaparin is not approved for use in patients receiving hemodialysis, where the half-life is doubled compared to healthy patients. If low-molecular-weight heparins must be used in patients with severely reduced kidney function, anti-Xa level monitoring should be considered in addition to dose adjustments.
Bivalirudin and argatroban are parenteral direct thrombin inhibitors used in the management of ACS and heparin-induced thrombocytopenia. Bivalirudin is primarily cleared by the kidneys requiring dose adjustments in patients with CrCl < 30 mL/min. Argatroban, which undergoes hepatic metabolism and clearance, may be the drug of choice among this class of antithrombotics in patients with advanced CKD.
Fondaparinux, the primary parental factor Xa inhibitor, undergoes extensive kidney elimination. Used in the treatment of ACS and VTE, including heparin-induced thrombocytopenia, it indirectly inhibits factor Xa via activation of antithrombin. In patients with CrCl 30–50 mL/min, the package insert for fondaparinux recommends judicious use and anti-Xa levels monitoring. Fondaparinux should be avoided in patients with CrCl < 30 mL/min.
Vitamin K antagonists, primarily warfarin, inhibit the hepatic synthesis of vitamin K-dependent coagulation factors II, VII, IX, and X, as well as proteins C and S. Warfarin’s use is ubiquitous in the management of ACS, VTE, AF, and heart valve replacement. Although it is hepatically metabolized and prescribing information does not recommend any adjustments in CKD, data suggest that patients with CKD require 10–20% lower doses. Given that these patients are at higher risk of bleeding complications, careful dosing and closer monitoring of international normalized ratio (INR) is advised.
Novel oral anticoagulants have now been developed; unlike warfarin, these agents are used in fixed doses, lack food interactions, require no laboratory monitoring, and have few drug interactions. Although more convenient, these new agents present different challenges due to their pharmacokinetic properties and inability for reversal, which can make bleeding complications, especially in susceptible patients with CKD, more difficult to manage.
Rivaroxaban and apixaban are direct oral factor Xa inhibitors indicated for stroke prevention in non-valvular AF, treatment of VTE, and VTE prophylaxis after knee and hip replacement surgery. One third of rivaroxaban is excreted by the kidneys. Apixaban has only 25% renal excretion, making it the safest of the novel anticoagulants in CKD. Dosing regimens for each of these agents vary based on the indication for use as well as the patient’s level of kidney function, age, weight, and concomitant use of P-glycoprotein inhibitors. Prior to using these novel anticoagulants in CKD, physicians should consult drug dosing guides.
Dabigatran is an oral direct thrombin inhibitor currently approved for stroke prevention in non-valvular AF and treatment of VTE. Dabigatran is about 80% cleared by the kidneys leading to increased drug exposure, decreased clearance, and amplified anticoagulation effects in patients with CKD. If this agent must be used in these patients, adjustments for kidney function must be made.
Guidelines endorse the use of novel oral anticoagulants instead of warfarin in patients with CrCl > 30 mL/min, especially if patients cannot maintain INRs in the therapeutic range. When choosing an oral anticoagulant, warfarin should be used in patients with severely reduced kidney function, given the ability to monitor anticoagulation and reverse the drug’s effects if bleeding occurs. In general, apixaban is preferred over rivaroxaban in CKD, and dabigatran, with the most kidney excretion, should be avoided.
In summary, providers should use prescribing information, guidelines, pharmacokinetic properties, reliable estimation of kidney function, and clinical judgment to optimize antiplatelet and antithrombotic drug therapy in CKD. Individualized factors such as stage of CKD, mode of administration, adherence, frequency of dosing, drug/drug interactions, drug/disease interactions, requirements for laboratory monitoring, cost, and patient/caretaker preference must also be considered.
- ».Basra SS, Tsai P, Lakkis NM. Safety and efficacy of antiplatelet and antithrombotic therapy in acute coronary syndrome in patients with chronic kidney disease. J Am Coll Cardiol. 2011;58(22):2263–2269. doi: 10.1016/j.jacc.2011.08.051. [DOI] [PubMed] [Google Scholar]
- ».Dapodanno D, Angiolillo DJ. Antithrombotic therapy in patients with chronic kidney disease. Circulation. 2012;125(21):2649–2661. doi: 10.1161/CIRCULATIONAHA.111.084996. [DOI] [PubMed] [Google Scholar]
- ».Harder S. Renal profiles of anticoagulants. J Clin Pharmacol. 2012;52(7):964–975. doi: 10.1177/0091270011409231. [DOI] [PubMed] [Google Scholar]
- ».Reinecke H, Engelbertz C, Schäbitz WR. Preventing stroke in patients with chronic kidney disease and atrial fibrillation: benefit and risks of old and new oral anticoagulants. Stroke. 2013;44(10):2935–2941. doi: 10.1161/STROKEAHA.113.001701. [DOI] [PubMed] [Google Scholar]
- ».Lane DA, Lip GYH. Use of the CHA2DS2-VASc and HAS-BLED scores to aid decision making for thromboprophylaxis in non-valvular AF. Circulation. 2012;126(7):860–865. doi: 10.1161/CIRCULATIONAHA.111.060061. [DOI] [PubMed] [Google Scholar]
IODINATED CONTRAST MEDIA
Diagnostic testing and interventions with intravascular iodinated contrast agents threaten kidney function. Contrast-enhanced studies including computed tomography (CT) imaging and angiography have become commonplace in healthcare, leading to increasing incidence of contrast-induced nephropathy (CIN). No consensus definition for CIN exists; however, in the absence of an alternate etiology, a rise in serum creatinine of 0.5 mg/dL or a 25% relative rise in creatinine is considered CIN if it occurs within 48 hours after contrast exposure.
CIN is generally described to be a transient, although not necessarily benign, condition. Among hospitalized patients, CIN is associated with longer inpatient stays, more complications, and higher one-year mortality. Evidence suggests that episodes of AKI, including those as a consequence of contrast exposure, contribute to long term loss of kidney function. Patients with moderate to severe baseline CKD who develop even mild or transient CIN have a significantly higher mortality rate than their counterparts without CIN. Judicious use of iodinated contrast-enhanced studies in high risk patients is an important precautionary step to reducing the incidence CIN. Patients with underlying CKD are most vulnerable to CIN. Other risk factors include advanced age, concomitant nephrotoxic drug use, diabetes mellitus, congestive heart failure, hypovolemia, acute severe hypotension, ST-segment elevation myocardial infarction, and hyperuricemia. Multiple risk factors increase the possibility of developing CIN. Individuals without any of these risk factors are at substantially lower risk of CIN.
Evidence is emerging that the potential risk for developing CIN varies depending on the contrast agent. Viscosity and osmolality are chemical characteristics that might be associated with the development of CIN. Animal studies suggest that highly viscous (low-osmolar) media decrease cortical and medullary blood flow and induce medullary hypoxia. Clinical trials have not yet been definitive in identifying the differential risk of non-iodinated low-osmolar versus iso-osmolar contrast agents. Preliminary evidence shows non-ionic iso-osmolar but highly viscous dimer contrast (iodixanol) used for angiography in high risk patients is less likely to induce CIN than non-ionic low-osmolar contrast. However, well-controlled trials have not shown a benefit in using non-ionic, low-osmolar, less viscous monomer or non-ionic, iso-osmolar iodixanol compared to standard contrast agents.
Whether intra-arterial contrast administration confers a higher risk than intravenous contrast remains controversial. Intra-arterial contrast is typically administered to patients undergoing coronary angiography who are more likely to have multiple risk factors for CIN and confounding hemodynamic instability, and are subject to microemboli during intra-arterial contrast administration.
Prevention strategies have been broadly targeted at proposed mechanisms for CIN, including renal medulla vasoconstriction with resultant renal tubule ischemia, direct tubular epithelial membrane toxicity, and reactive oxygen species production. Using N-acetylcysteine as an anti-oxidant agent has had mixed results in terms of CIN prevention. Urinary alkalinization via sodium bicarbonate administration may also inhibit reactive oxygen species, but studies evaluating its efficacy in preventing CIN have been inconclusive. Recently, BOSS (Bicarbonate or Saline Study) was terminated early due to higher death rates in patients receiving normal saline compared to those receiving sodium bicarbonate one hour prior to and during coronary angiography, although AKI rates were similar between groups. Attempts to employ diuretics to augment medullary blood flow and urinary output and thereby reduce contrast media contact time with the tubule lumen have been unsuccessful. A recent meta-analysis examining the role of statins in CIN prevention showed that statin use may reduce serum creatinine but does not prevent CIN or the need for renal replacement therapy. The most commonly accepted prevention strategy is normal saline hydration, which likely offers protection by decreasing contrast media concentration in the renal tubules and enhancing urine flow.
When the clinical need arises to employ iodine-based contrast imaging, providers should first carefully analyze the indication for contrast enhancement based on pre-test probability for the suspected diagnosis. Patients should be screened for CIN risk factors, and the utility of non-contrast enhanced imaging modalities in high risk patients should be considered carefully. In the absence of alterative diagnostic or therapeutic options, preventative measures should include adequate hydration with normal saline and discontinuation of nephrotoxic medications. The lowest dose of contrast should be used and every effort made to avoid serial contrast studies. Serum creatinine monitoring postcontrast exposure is prudent, particularly in patients using drugs that are cleared by the kidneys and that have narrow therapeutic indices.
- ».Nyman U, Almén T, Jacobsson B, Aspelin P. Are intravenous injections of contrast media really less nephrotoxic than intra-arterial injections? Eur Radiol. 2012;22(6):1366–1371. doi: 10.1007/s00330-011-2371-4. [DOI] [PubMed] [Google Scholar]
- ».Reddan D, Laville M, Garovic VD. Contrast-induced nephropathy and its prevention: what do we really know from evidence-based findings? J Nephrol. 2009;22(3):333–351. [PubMed] [Google Scholar]
- ».Solomon R, Dauerman HL. Contrast-Induced Acute Kidney Injury. Circulation. 2010;122(23):2451–2455. doi: 10.1161/CIRCULATIONAHA.110.953851. [DOI] [PubMed] [Google Scholar]
- ».Solomon RJ, Natarajan MK, Doucet S, et al. Cardiac Angiography in Renally Impaired Patients (CARE) Study: A Randomized Double-Blind Trial of Contrast-Induced Nephropathy in Patients With Chronic Kidney Disease. Circulation. 2007;115(25):3189–3196. doi: 10.1161/CIRCULATIONAHA.106.671644. [DOI] [PubMed] [Google Scholar]
- ».Voeltz MD, Nelson MA, McDaniel MC, Manoukian SV. The Important Properties of Contrast Media: Focus on Viscosity. J Invasive Cardiol. 2007;19(3):1A–9A. [PubMed] [Google Scholar]
GADOLUNIUM-BASED CONTRAST MEDIA
Nephrogenic systemic fibrosis (NSF) is a condition that occurs only in patients with CKD after exposure to gadolinium-based contrast agents (GBCA), which are administered with magnetic resonance imaging (MRI). NSF most commonly manifests as pruritic or painful skin thickening weeks to months after GBCA exposure, but multiorgan fibrosis accompanied by significantly higher morbidity and increased mortality may occur. Typically chronic and unremitting, NSF may slow or reverse with improvement of AKI or kidney transplantation.
Risk of developing NSF is related to degree of reduced kidney function, chemical properties of the gadolinium contrast, and possibly dose of GBCA. Patients at highest risk are chronic kidney failure patients treated by dialysis; however cases have been reported among those with advanced CKD or AKI not requiring dialysis. Less thermodynamically stable gadolinium compounds such as Omniscan or Magnevist have been implicated in a greater proportion of NSF cases than more thermodynamically stable gadolinium agents such as Opti-MARK, ProHance, MultiHance, Dotarem, Ablavar and Eovist. Risk may be gadolinium dose-dependent. The pathophysiology of NSF is not well understood and no clear prevention strategies have been identified, except GBCA avoidance in high-risk patients. Since 2007, when the FDA issued a warning regarding the risk of NSF from GBCA use, fewer NSF cases have been reported. Clinicians should consider alternative imaging modalities in high risk patients. If GBCA must be used in patients with reduced kidney function, a more stable gadolinium compound should be used at the lowest possible dose. Hemodialysis therapy should be considered after GBCA use in patients with ESRD or advanced CKD who have a vascular access. However, in patients without access or less severe CKD, the utility of hemodialysis as a means to mitigate the risk of NSF is unknown and therefore not recommended.
- ».Daftari Besheli L, Aran S, Shaqdan K, Kay J, Abujudeh H. Current status of nephrogenic systemic fibrosis. Clin Radiol. 2014 Jul;69(7):661–668. doi: 10.1016/j.crad.2014.01.003. [DOI] [PubMed] [Google Scholar]
CONCLUSION
CKD is notable for the wide array of potential threats to patient safety found in delivery of standard care. Providers face many challenges in safeguarding the care of CKD patients given the frequent healthcare encounters that these patients have over the course of their chronic illness. The complexity of CKD requires collaboration between providers, attention to the unique pharmacologic considerations needed with reduced kidney function, and continuous assessment of the costs and benefits of any therapeutic intervention or diagnostic test. Ensuring the safety of care delivered to patients with is an essential and powerful step toward reducing poor outcomes—including accelerated loss of kidney function, progression to ESRD, and death—in this high risk population. Maintaining patient safety in CKD is likely to be as important as any currently available therapy for the disease, with just as great a benefit.
ACKNOWLEDGEMENTS
Support: None.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no relevant financial interests.