Abstract
Afucosylated antibodies potentiate natural killer (NK) cell-mediated antibody-dependent cellular cytotoxicity (ADCC) by enhancing signaling pathways and cellular processes, which in turn, increases cytotoxic potential. Importantly, a better understanding of these processes and properties will aid in exploiting them to help design therapeutic antibodies and strategies that may be of the greatest benefit to patients.
Keywords: afucosylated therapeutic antibodies, antibody-dependent cellular cytotoxicity, cellular processes, natural killer cells, obinutuzumab, rituximab, signaling, trastuzumab
Antibody-dependent cellular cytotoxicity (ADCC) has been implicated as one of the mechanisms by which therapeutic antibodies mediate their antitumor effects.1 This process is facilitated by FcγRIIIa expressed on NK cells or macrophages, and the Fc portion of the tumor-bound antibody.2 Upon engagement between FcγRIIIa and the antibody, a series of biochemical signaling events are initiated that lead to the secretion of lytic molecules toward the target cell.3
The importance of promoting ADCC in tumor eradication has been documented in numerous studies. In preclinical studies, mice deficient for the FcgR chain are less responsive to therapeutic antibody treatment relative to wild-type mice.2 Further, mice treated with an “effectorless” form of antibody that does not interact with the Fc receptor exhibit lower antitumor activity compared to mice treated with the unmodified form.2 In some clinical studies, though not in all, patients with the high affinity allele of FcγRIIIa enjoy a better response to therapeutic antibodies.4 The basis for such clinical inconsistencies is not yet known, but efforts to increase ADCC through modification of the Fc portion of the antibody have proceeded nonetheless. Toward this end, removing the fucose moiety on the oligosaccharide chain of asparagine 297 yields an increase in the affinity between FcγRIIIa and the antibody, and an overall increase in ADCC.5 These observations prompted the development of obinutuzumab, an afucosylated variant of rituximab (an anti-CD20 antibody).6 Obinutuzumab has been recently approved by health authorities because of its improved efficacy, relative to rituximab, in chronic lymphocytic leukemia patients.7
FcγRIIIa is also expressed on macrophages and can facilitate ADCC,2 as well as antibody-dependent phagocytosis (ADP) to drive therapeutic antibody-mediated tumor clearance in vivo.8 Afucosylated antibodies can enhance these processes for target cell clearance;9 however, the mechanisms accounting for such enhancement remain unknown. Because macrophages use signaling pathways similar to those responsible for ADCC in NK cells,9 understanding mechanisms operating in NK cells may lend insight into the mechanisms behind the enhancement in antibody-mediated macrophage antitumor activities.
Our studies focused on understanding the effect of increased affinity between afucosylated antibodies and FcγRIIIa on the molecular and cellular mechanisms, as well as cytotoxic characteristics, in NK cells (Fig. 1). We used two different sets of antibodies (afucosylated trastuzumab/trastuzumab, and obinutuzumab/rituximab) to learn that afucosylated antibodies increase early FcγRIIIa signaling, as well as signaling through the Vav1, MAPK, and PI3K pathways (Fig. 1).10 Consistent with those observations, afucosylated trastuzumab and obinutuzumab enhanced actin rearrangement and degranulation10 (Fig. 1), 2 cellular processes essential for cytotoxicity.
Figure 1.
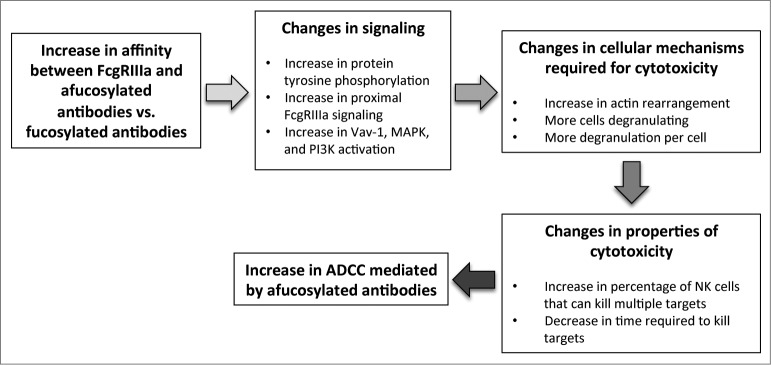
The increase in affinity between FcγRIIIa and afucosylated antibodies (versus fucosylated antibodies) results in changes to signaling pathways, cellular mechanisms, and cytotoxic properties to enhance ADCC. Removing the fucose moiety on the oligosaccharide chain of asparagine 297 on the antibody leads to an increase in affinity between FcγRIIIa and the antibody, which ultimately results in increased ADCC. Our studies show this affinity change promotes signaling, inclusive of increases in overall protein tyrosine phosphorylation, proximal FcγRIIIa signaling components, and the Vav-1, MAPK, and PI3K signaling pathways. This enhancement of signaling increases the number of cells activated, actin rearrangement, and degranulation, which in turn promote cytotoxic properties. These changes include an increase in the cytotoxic rate of individual NK cells and the percentage of NK cells that can kill multiple targets. Together, these enhancements to signaling, cellular cytotoxic mechanisms, and cytotoxic properties afforded by the increase in affinity between FcγRIIIa and afucosylated antibodies serve to promote ADCC.
As a functional readout of these alterations in molecular and cellular mechanisms, we developed a microscope-based cytotoxicity assay that permits the measurement of cytotoxicity while observing the interaction between NK and target cells. Our experiments disclosed that afucosylated antibodies increase the cytotoxic potential of individual NK cells by increasing the rate at which they lyse targets (Fig. 1).10 In addition, afucosylated antibodies enhance the cytotoxic potential of the entire NK cell population by increasing the number of cells that can perform multiple killing events (Fig. 1).10 Thus, afucosylated antibodies increase NK cell-mediated ADCC by potentiating signaling pathways to promote cellular processes required for cytotoxicity, which increases the cytotoxic potential of individual NK cells and the whole NK cell population (Fig. 1).
In light of the increasing focus in the pharmaceutical industry on the use of combined therapeutics, a better understanding of these mechanisms may aid in the design of approaches to ensure that afucosylated antibodies remain effective in combination with other therapeutics. Specifically, in the context of the development and use of small molecule inhibitors of components of the MAPK, PI3K, and other pathways important for cancer growth and survival, concerns may arise that these molecules may inadvertently inhibit signaling in immune cells and thus diminish or even disable ADCC. In instances where signaling is diminished by a molecule that is co-administered with a therapeutic antibody, it obviously will be advantageous to ensure that the therapeutic antibody is itself maximally capable of driving FcγRIIIa-dependent signaling; hence the advantage presented by afucosylated therapeutic antibodies.
Another advantage of afucosylated antibodies is the observation that lower concentrations of these molecules, relative to the fucosylated versions, are required to generate the biochemical events required for adequate cytotoxicity. Our studies show that approximately 2 to 20 times more trastuzumab is required to exhibit the same phospho-tyrosine signature as afucosylated trastuzumab,10 implying that the efficacious dose of an afucosylated antibody may be less than the efficacious dose of its fucosylated equivalent, with the attendant potential for greater safety. This wide range of donor-dependent response, along with our observation that the number of NK cells capable of performing serial killing varies between different target cell lines,10 indicates that unknown factors on NK or target cells can modulate FcγRIIIa-mediated stimulation. Molecules and mechanisms that may be operative in this context include variation in the expression levels of activating receptors like NKG2D, which, when engaged after the NK cell is bound to the target cell through the antibody-Fc receptor, leads to enhanced signaling in a mode akin to a functional co-receptor. Similarly, variation in the expression level of the target antigen, activating/inhibitory ligands, and/or adhesion molecules on different tumor types may also influence cytotoxic properties.
These and future studies will serve to advance the understanding of potentiating therapeutic antibodies, as a mono-therapeutic or in combination with other therapeutics, in a way to exploit their signaling mechanisms and cytotoxic properties to help design and guide strategies that may be of the greatest benefit to patients.
Disclosure of Potential Conflicts of Interest
All authors are employees of Genentech, Inc., and have ownership interest in Roche.
References
- 1.Hudis CA. Trastuzumab–mechanism of action and use in clinical practice. N Engl J Med 2007; 357:39-51; PMID:; http://dx.doi.org/ 10.1056/NEJMra043186 [DOI] [PubMed] [Google Scholar]
- 2.Clynes RA, Towers TL, Presta LG, Ravetch JV.. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med 2000; 6:443-6; PMID:; http://dx.doi.org/ 10.1038/74704 [DOI] [PubMed] [Google Scholar]
- 3.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol 2008; 9:495-502; PMID:; http://dx.doi.org/ 10.1038/ni1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mellor JD, Brown MP, Irving HR, Zalcberg JR, Dobrovic A.. A critical review of the role of Fc gamma receptor polymorphisms in the response to monoclonal antibodies in cancer. J Hematol Oncol 2013; 6:1; PMID:; http://dx.doi.org/ 10.1186/1756-8722-6-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Junttila TT, Parsons K, Olsson C, Lu Y, Xin Y, Theriault J, Crocker L, Pabonan O, Baginski T, Meng G et al.. Superior in vivo efficacy of afucosylated trastuzumab in the treatment of HER2-amplified breast cancer. Cancer Res 2010; 70:4481-9; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-3704 [DOI] [PubMed] [Google Scholar]
- 6.Mossner E, Brunker P, Moser S, Puntener U, Schmidt C, Herter S, Grau R, Gerdes C, Nopora A, van Puijenbroek E et al.. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood 2010; 115:4393-402; PMID:; http://dx.doi.org/ 10.1182/blood-2009-06-225979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, Chagorova T, De La Serna J, Dilhuydy MS, Opat S et al.. Head-To-Head Comparison Of Obinutuzumab (GA101) Plus Chlorambucil (Clb) Versus Rituximab Plus Clb In Patients With Chronic Lymphocytic Leukemia (CLL) and Co-Existing Medical Conditions (Comorbidities): Final Stage 2 Results Of The CLL11 Trial. New Orleans, LA: ASH Annual Meeting and Exposition; 2013. [Google Scholar]
- 8.Braster R, O'Toole T, van Egmond M.. Myeloid cells as effector cells for monoclonal antibody therapy of cancer. Methods 2014; 65:28-37; PMID:; http://dx.doi.org/ 10.1016/j.ymeth.2013.06.020 [DOI] [PubMed] [Google Scholar]
- 9.Herter S, Birk MC, Klein C, Gerdes C, Umana P, Bacac M.. Glycoengineering of therapeutic antibodies enhances monocyte/macrophage-mediated phagocytosis and cytotoxicity. J Immunol 2014; 192:2252-60; PMID:; http://dx.doi.org/ 10.4049/jimmunol.1301249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu SD, Chalouni C, Young JC, Junttila TT, Sliwkowski MX, Lowe JB.. Afucosylated antibodies increase activation of FcgammaRIIIa-dependent signaling components to intensify processes promoting ADCC. Cancer Immunology Research, 2015 Feb; 3(2):173-83; PMID: 25387893; http://dx.doi.org/ 10.1158/2326-6066.CIR-14-0125. [DOI] [PubMed] [Google Scholar]


