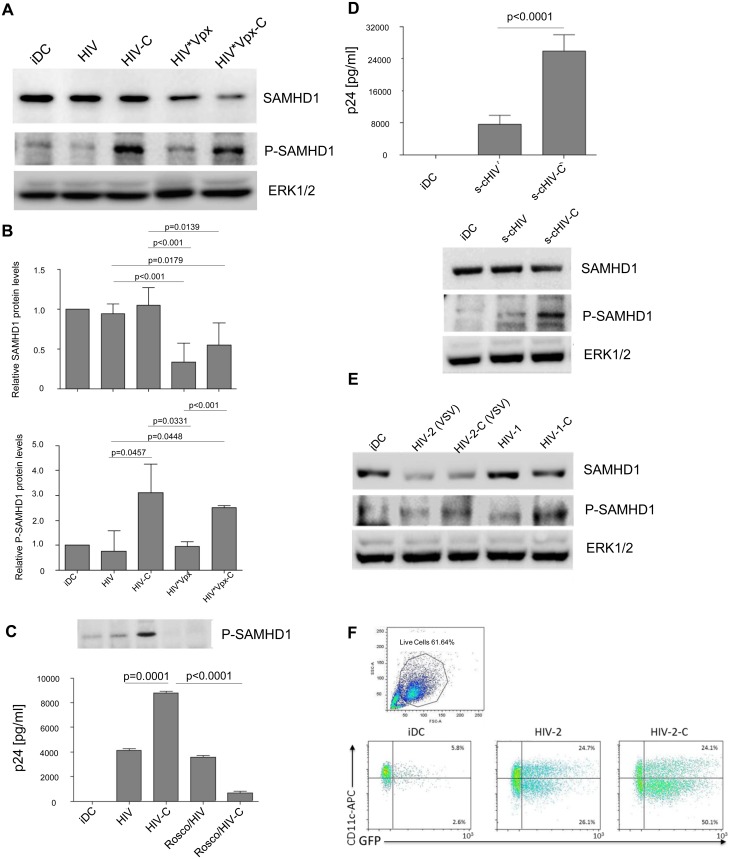
Fig 2. SAMHD1 is highly phosphorylated in HIV-C-DCs.
(A) 1 st panel: SAMHD1 protein expression is unaltered in DCs incubated with HIV or HIV-C compared to iDCs (lanes 1–3), while degradation of SAMHD1 can be detected after 24h when cells were incubated with HIV*Vpx and HIV*Vpx-C (lanes 4 and 5). 2 nd panel: Compared to iDCs (lane 1), and DCs exposed to non-opsonized HIV (HIV, lane 2) or HIV*Vpx (HIV*Vpx, lane 4), SAMHD1 phosphorylation at SAMHD1 residue T592 is highly increased after 4h in C-opsonized HIV and HIV*Vpx-DCs (HIV-C and HIV*Vpx-C, lanes 3 and 5). 3 rd panel: Protein expression of non-phosphorylated ERK1/2 was used as control for proper protein loading of the samples. Western Blots were performed using lysates of DCs loaded with differentially opsonized HIV (HIV and HIV*Vpx) for 24h from 5 donors and one representative example is shown. (B) SAMHD1 (left chart) and P-SAMHD1 (right chart) protein expression values (from Western blot experiments) were normalized from 3 donors as stated in the Results section by ImageJ quantification. Protein expression of ERK1/2 was used as loading control and additionally relative comparison to iDC protein expression was performed. (C) Inhibition of SAMHD1 phosphorylation abrogates HIV-C-mediated DC infection. DCs were incubated or not for 24h with the specific CDK2 inhibitor Roscovitine (50μM) before infection with HIV or HIV-C for another 24h. Immuno-blot analyses of iDCs, HIV-DCs, HIV-C-DCs, Roscovitine-HIV-DCs and Roscovitine-HIV-C-DCs were performed (upper panel). No SAMHD1 T592 phosphorylation signal was detected in DCs pre-treated with Roscovitine (lane 4, 5), while again C-opsonized HIV (HIV-C) mediated SAMHD1 T592 phosphorylation (lane 3). Additionally, infection assays were performed in absence and presence of Roscovidine. As shown by p24 ELISA on the chart, HIV-C significantly enhanced DC infection compared to non-opsonized HIV, while pre-incubation with Roscovitine significantly blocked HIV-C-mediated DC infection. For these analyses, cells from two donors and three different virus strains were used. (D) Productive DC infection and SAMHD1 phosphorylation are also enhanced using complement-opsonized single-cycle HIV-1. Analyses of infection and SAMHD1 phosphorylation were repeated in three independent experiments (in triplicates for infection assays) using single-cycle HIV-1, which was opsonized or not with complement (s-cHIV, s-cHIV-C). These analyses revealed that the highly significant (p<0.0001) enhancement of DC infection by HIV-C does not depend on multiple rounds of infection, but is also achieved using s-cHIV-C (left). As observed with replicating HIV, also s-cHIV only caused a low-level productive infection in DCs (left). Furthermore, s-cHIV-C mediated SAMHD1 phosphorylation in contrast to the non-opsonized HIV-1 preparation (right). (E) HIV-2 (VSV) degrades SAMHD1. 1 st panel: SAMHD1 protein expression is similar in control DCs (iDC; lane 1), or non- and C-opsonized HIV-1 (HIV-1, HIV-1-C; lanes 4 and 5) after 24h. In contrast, non-opsonized and C-opsonized HIV-2 (HIV-2, HIV-2-C; lanes 2 and 3) caused degradation of total SAMHD1 protein expression levels. 2 nd panel: T592 phosphorylation of SAMHD1 was detected after 3h in C-opsonized HIV-1 loaded DCs (HIV-1-C; lane 5) and at a lower level in C-opsonized HIV-2 treated DCs (HIV-2-C; lane 3). Similar to previous results (Fig 2A) no phosphorylation of SAMHD1 was observed in immature DCs (iDC; lane 1) as well as in cell lysates from non-opsonized HIV-1 or HIV-2 treated DCs (HIV-1, HIV-2; lanes 2 and 4). Results of one representative donor are shown in the figure. The experiment was repeated in 3 different donors. (F) HIV-2 (VSV) and HIV-2-C (VSV) efficiently infect DCs. FACS analyses of live, HIV-2-exposed DCs revealed productive infection with both HIV-2 (VSV) and HIV-2-C (VSV). Nevertheless, C-opsonization of HIV-2 mediated a 1.5-fold enhancement of infection in three independent donors. One representative FACS analysis is depicted.