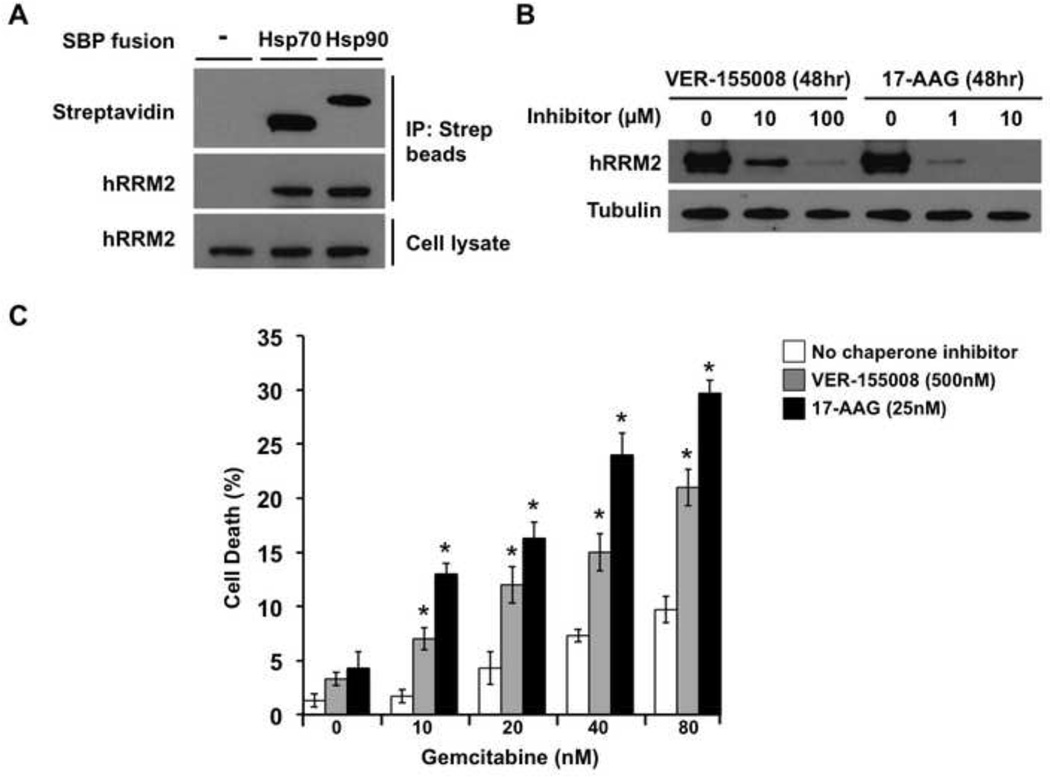
Figure 7. Ribonucleotide reductase is a client of Hsp70 and Hsp90 in human breast cancer cells.
A) MCF-7 human breast cancer cells were transiently transfected with no DNA (control) or plasmids expressing SBP-tagged Hsp70 or Hsp90. After 48 h recovery in media, cells were lysed and pull-down was performed with streptavidin-conjugated magnetic beads. Captured proteins were separated by SDS-PAGE, transferred and probed with streptavidin-HRP or anti-hRRM2 antibody and secondary antibody HRP conjugate.
B) MCF-7 cells grown to 50% confluence were treated either with DMSO, Hsp70 inhibitor VER-155008, or Hsp90 inhibitor 17-AAG for 48 h. Cells were lysed and hRRM2 levels were determined by Western blot with anti-hRRM2 antibody. Equal loading was determined using anti-tubulin immunoreactivity.
C) MCF-7 cells were incubated without chaperone inhibitors or with subtoxic concentrations of VER-155008 to inhibit Hsp70 or 17-AAG to inhibit Hsp90. After 24 h, gemcitabine was added at the indicated concentrations. After another 24 h, cell death was determined by Trypan Blue dye exclusion assay. Data shown are mean ± SEM of three replicates (* P < 0.05 compared to no chaperone inhibitor, t-test).