Figure 4. IRE1 RIDD Contributes to Cell Death.
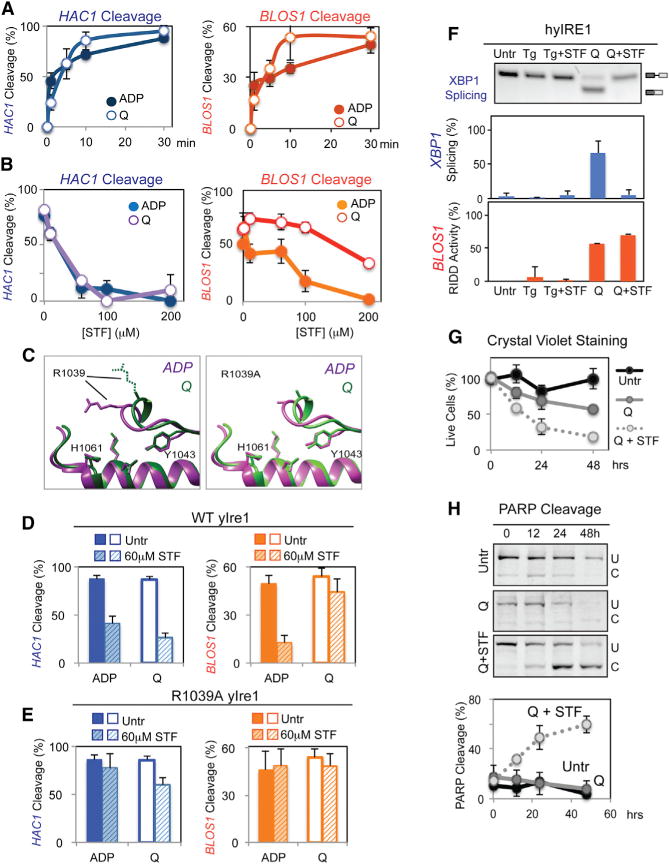
(A) Cleavage of HAC1 (left) or BLOS1 RNA (right) with WT yIre1 (1 μM) activated with ADP (2 μM) (closed circle) or Q (100 μM) (open circle). Error bars in this figure were calculated from at least three independent experiments.
(B) Nuclease reactions were performed with varying concentrations of STF-083010 (STF), an inhibitor, on WT yIre1 (1 μM) activated with either ADP (2 μM) (closed circles) or Q (100 μM) (open circles).
(C) The core RNase catalytic center between oligomerized Ire1, the ADP activated form “ADP” (purple) (Korennykh et al., 2009) and Q activated “Q” (green) yIre1 (Wiseman et al., 2010) is similar at the molecular level, except for the spatial orientation of the R1039 residue. The right panel shows structure predictions of R1039A yIre1 activated by ADP (purple) or Q (green).
(D and E) HAC1 or RIDD cleavage by (D) WT yIre1 (1 μM) or (E) R1039A yIRE1 was activated by Q (100 μM) or ADP (2 μM) with and without STF (60 μM) for 30 min.
(F) STF inhibited XBP1 mRNA splicing but not RIDD for Q-activated hybrid IRE1 (hyIRE1) in vivo with or without STF (60 μM) for 2 hr.
(G and H) Preferential inhibition of XBP1 splicing but not RIDD in hyIRE1 induced (G) cell death and (H) apoptosis.
