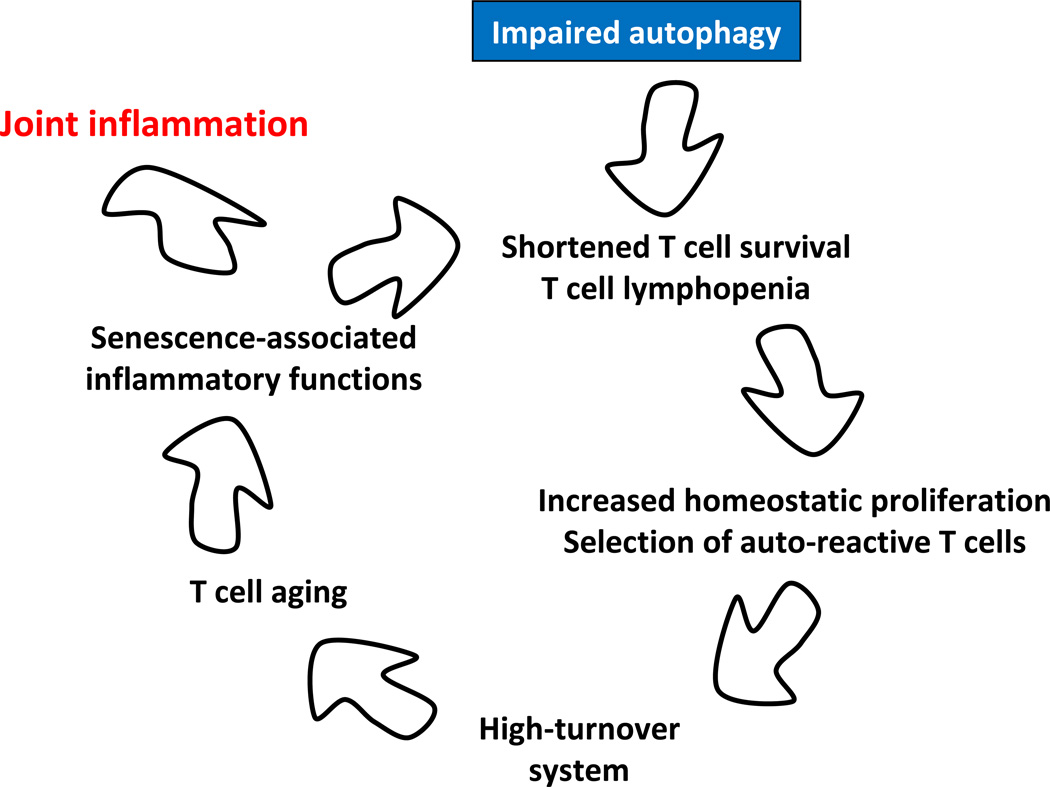
Figure 7. Defective autophagy as a risk factor for autoimmunity – a hypothetical model.
In response to antigen and danger signals, lymphocytes mobilize autophagy as a basal mechanism to alleviate stress and fulfill the energy demands associated with clonal expansion. T cells from RA patients fail to upregulate autophagic activity, rendering them susceptible to apoptosis. Evolving lymphopenia triggers compensatory homeostatic proliferation to make up for the T cell loss. Proliferating T cells are selected on autoantigens, biasing the T cell repertoire towards recognition of self. Chronic T cell loss sustains a high-turnover system, eventually causing T cell aging. Prematurely aged T cells acquire the so-called senescence-associated secretory phenotype (SASP) and release massive amounts of inflammatory mediators. The end result is a host predisposed to tissue inflammation. Synovia-specific antigens may direct hyperinflammatory T cells towards the joint.