Abstract
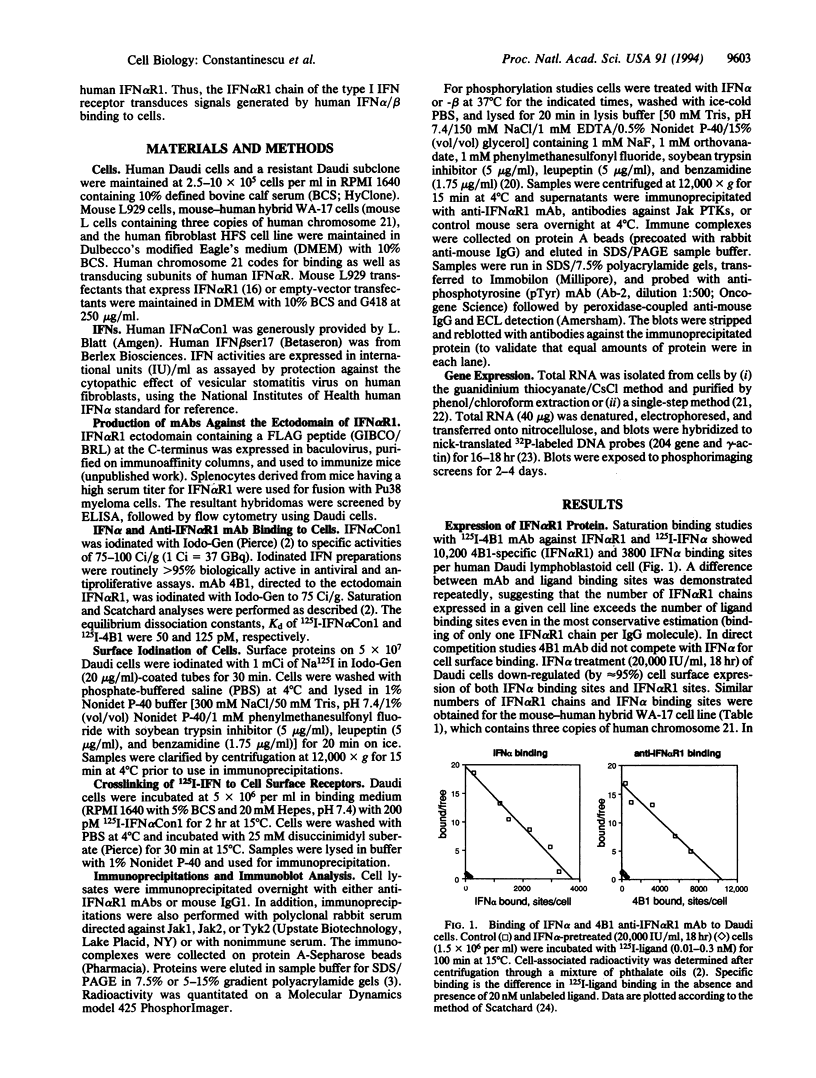
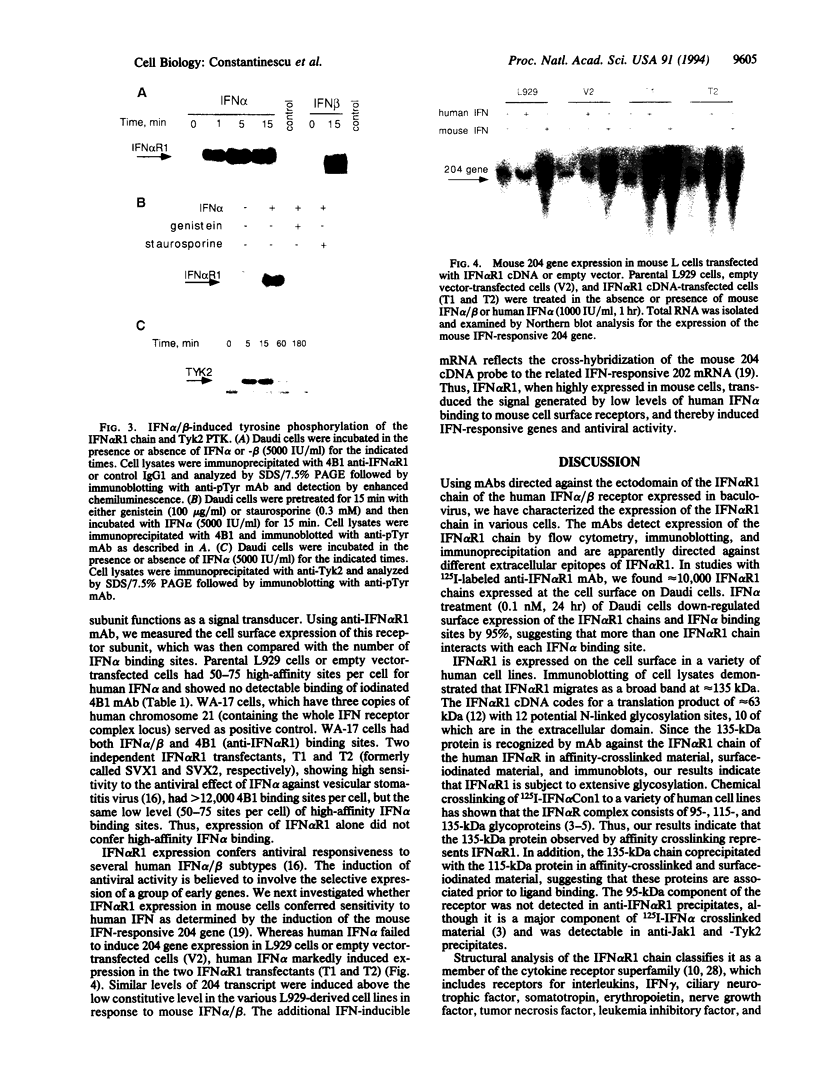
A previously cloned cDNA encodes one subunit of the human interferon alpha/beta receptor (IFN alpha R), denoted IFN alpha R1. To study the expression and signaling of IFN alpha R1, we used monoclonal antibodies (mAbs) generated against the baculovirus-expressed ectodomain of IFN alpha R1. Immunoprecipitation and immunoblotting of lysates from a variety of human cell lines showed that IFN alpha R1 has an apparent molecular mass of 135 kDa. Binding analysis with 125I-labeled mAb demonstrated high levels of cell surface expression of IFN alpha R1 in human cells and in mouse cells transfected with IFN alpha R1 cDNA, whereas no cross-reactivity was observed in control mouse L929 cells expressing only the endogenous mouse receptor. The subunit was rapidly down-regulated by IFN alpha (80% decrease within 2 hr) and degraded upon internalization. The IFN alpha R1 chain appeared to be constitutively associated with the 115-kDa subunit of the IFN alpha/beta receptor, since the mAbs coprecipitated this protein. IFN alpha/beta treatment induced tyrosine phosphorylation of IFN alpha R1 within 1 min, with kinetics paralleling that of the IFN-activated protein-tyrosine kinases Jak1 and Tyk2. Ligand-induced tyrosine phosphorylation of IFN alpha R1 was blocked by the kinase inhibitors genistein or staurosporine. Although IFN alpha R1 cDNA-transfected mouse cells expressed high levels of this subunit when compared with empty vector-transfected cells the number of binding sites for human IFN alpha (50-75 sites per cell) was not increased. Human IFN alpha induced the expression of a mouse IFN alpha/beta-responsive gene (the 204 gene) in mouse L929 cells transfected with the IFN alpha R1 cDNA, but not in mock-transfected cells. These results suggest that the IFN alpha R1 subunit acts as a species-specific signal transduction component of the IFN alpha/beta receptor complex.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazan J. F. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit P., Maguire D., Plavec I., Kocher H., Tovey M., Meyer F. A monoclonal antibody to recombinant human IFN-alpha receptor inhibits biologic activity of several species of human IFN-alpha, IFN-beta, and IFN-omega. Detection of heterogeneity of the cellular type I IFN receptor. J Immunol. 1993 Feb 1;150(3):707–716. [PubMed] [Google Scholar]
- Branca A. A., Faltynek C. R., D'Alessandro S. B., Baglioni C. Interaction of interferon with cellular receptors. Internalization and degradation of cell-bound interferon. J Biol Chem. 1982 Nov 25;257(22):13291–13296. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Choubey D., Snoddy J., Chaturvedi V., Toniato E., Opdenakker G., Thakur A., Samanta H., Engel D. A., Lengyel P. Interferons as gene activators. Indications for repeated gene duplication during the evolution of a cluster of interferon-activatable genes on murine chromosome 1. J Biol Chem. 1989 Oct 15;264(29):17182–17189. [PubMed] [Google Scholar]
- Colamonici O. R., Pfeffer L. M., D'Alessandro F., Platanias L. C., Gregory S. A., Rosolen A., Nordan R., Cruciani R. A., Diaz M. O. Multichain structure of the IFN-alpha receptor on hematopoietic cells. J Immunol. 1992 Apr 1;148(7):2126–2132. [PubMed] [Google Scholar]
- Colamonici O. R., Porterfield B., Domanski P., Constantinescu S., Pfeffer L. M. Complementation of the interferon alpha response in resistant cells by expression of the cloned subunit of the interferon alpha receptor. A central role of this subunit in interferon alpha signaling. J Biol Chem. 1994 Apr 1;269(13):9598–9602. [PubMed] [Google Scholar]
- Donella-Deana A., Marin O., Brunati A. M., Cesaro L., Piutti C., Pinna L. A. Phosphorylated residues as specificity determinants for an acidophilic protein tyrosine kinase. A study with src and cdc2 derived phosphopeptides. FEBS Lett. 1993 Sep 13;330(2):141–145. doi: 10.1016/0014-5793(93)80260-2. [DOI] [PubMed] [Google Scholar]
- Friedman R. L., Stark G. R. alpha-Interferon-induced transcription of HLA and metallothionein genes containing homologous upstream sequences. Nature. 1985 Apr 18;314(6012):637–639. doi: 10.1038/314637a0. [DOI] [PubMed] [Google Scholar]
- Fu X. Y. A transcription factor with SH2 and SH3 domains is directly activated by an interferon alpha-induced cytoplasmic protein tyrosine kinase(s). Cell. 1992 Jul 24;70(2):323–335. doi: 10.1016/0092-8674(92)90106-m. [DOI] [PubMed] [Google Scholar]
- Hemmi S., Böhni R., Stark G., Di Marco F., Aguet M. A novel member of the interferon receptor family complements functionality of the murine interferon gamma receptor in human cells. Cell. 1994 Mar 11;76(5):803–810. doi: 10.1016/0092-8674(94)90355-7. [DOI] [PubMed] [Google Scholar]
- Mouchel-Vielh E., Lutfalla G., Mogensen K. E., Uzé G. Specific antiviral activities of the human alpha interferons are determined at the level of receptor (IFNAR) structure. FEBS Lett. 1992 Nov 30;313(3):255–259. doi: 10.1016/0014-5793(92)81204-y. [DOI] [PubMed] [Google Scholar]
- Müller M., Briscoe J., Laxton C., Guschin D., Ziemiecki A., Silvennoinen O., Harpur A. G., Barbieri G., Witthuhn B. A., Schindler C. The protein tyrosine kinase JAK1 complements defects in interferon-alpha/beta and -gamma signal transduction. Nature. 1993 Nov 11;366(6451):129–135. doi: 10.1038/366129a0. [DOI] [PubMed] [Google Scholar]
- Novick D., Cohen B., Rubinstein M. The human interferon alpha/beta receptor: characterization and molecular cloning. Cell. 1994 May 6;77(3):391–400. doi: 10.1016/0092-8674(94)90154-6. [DOI] [PubMed] [Google Scholar]
- Pearson R. B., Kemp B. E. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- Pfeffer L. M., Eisenkraft B. L., Reich N. C., Improta T., Baxter G., Daniel-Issakani S., Strulovici B. Transmembrane signaling by interferon alpha involves diacylglycerol production and activation of the epsilon isoform of protein kinase C in Daudi cells. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7988–7992. doi: 10.1073/pnas.88.18.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer L. M., Stebbing N., Donner D. B. Cytoskeletal association of human alpha-interferon-receptor complexes in interferon-sensitive and -resistant lymphoblastoid cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3249–3253. doi: 10.1073/pnas.84.10.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer L. M., Strulovici B., Saltiel A. R. Interferon-alpha selectively activates the beta isoform of protein kinase C through phosphatidylcholine hydrolysis. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6537–6541. doi: 10.1073/pnas.87.17.6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platanias L. C., Pfeffer L. M., Cruciani R., Colamonici O. R. Characterization of the alpha subunit of the IFN-alpha receptor. Evidence of N- and O-linked glycosylation and association with other surface proteins. J Immunol. 1993 Apr 15;150(8 Pt 1):3382–3388. [PubMed] [Google Scholar]
- Puissant C., Houdebine L. M. An improvement of the single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Biotechniques. 1990 Feb;8(2):148–149. [PubMed] [Google Scholar]
- Reich N., Evans B., Levy D., Fahey D., Knight E., Jr, Darnell J. E., Jr Interferon-induced transcription of a gene encoding a 15-kDa protein depends on an upstream enhancer element. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6394–6398. doi: 10.1073/pnas.84.18.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler C., Shuai K., Prezioso V. R., Darnell J. E., Jr Interferon-dependent tyrosine phosphorylation of a latent cytoplasmic transcription factor. Science. 1992 Aug 7;257(5071):809–813. doi: 10.1126/science.1496401. [DOI] [PubMed] [Google Scholar]
- Soh J., Donnelly R. J., Kotenko S., Mariano T. M., Cook J. R., Wang N., Emanuel S., Schwartz B., Miki T., Pestka S. Identification and sequence of an accessory factor required for activation of the human interferon gamma receptor. Cell. 1994 Mar 11;76(5):793–802. doi: 10.1016/0092-8674(94)90354-9. [DOI] [PubMed] [Google Scholar]
- Stahl N., Boulton T. G., Farruggella T., Ip N. Y., Davis S., Witthuhn B. A., Quelle F. W., Silvennoinen O., Barbieri G., Pellegrini S. Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6 beta receptor components. Science. 1994 Jan 7;263(5143):92–95. doi: 10.1126/science.8272873. [DOI] [PubMed] [Google Scholar]
- Stahl N., Yancopoulos G. D. The alphas, betas, and kinases of cytokine receptor complexes. Cell. 1993 Aug 27;74(4):587–590. doi: 10.1016/0092-8674(93)90506-l. [DOI] [PubMed] [Google Scholar]
- Taga T., Kishimoto T. Cytokine receptors and signal transduction. FASEB J. 1992 Dec;6(15):3387–3396. doi: 10.1096/fasebj.6.15.1334470. [DOI] [PubMed] [Google Scholar]
- Uzé G., Lutfalla G., Bandu M. T., Proudhon D., Mogensen K. E. Behavior of a cloned murine interferon alpha/beta receptor expressed in homospecific or heterospecific background. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4774–4778. doi: 10.1073/pnas.89.10.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzé G., Lutfalla G., Gresser I. Genetic transfer of a functional human interferon alpha receptor into mouse cells: cloning and expression of its cDNA. Cell. 1990 Jan 26;60(2):225–234. doi: 10.1016/0092-8674(90)90738-z. [DOI] [PubMed] [Google Scholar]
- Vanden Broecke C., Pfeffer L. M. Characterization of interferon-alpha binding sites on human cell lines. J Interferon Res. 1988 Dec;8(6):803–811. doi: 10.1089/jir.1988.8.803. [DOI] [PubMed] [Google Scholar]
- Velazquez L., Fellous M., Stark G. R., Pellegrini S. A protein tyrosine kinase in the interferon alpha/beta signaling pathway. Cell. 1992 Jul 24;70(2):313–322. doi: 10.1016/0092-8674(92)90105-l. [DOI] [PubMed] [Google Scholar]
- Wang C., Constantinescu S. N., MacEwan D. J., Strulovici B., Dekker L. V., Parker P. J., Pfeffer L. M. Interferon alpha induces protein kinase C-epsilon (PKC-epsilon) gene expression and a 4.7-kb PKC-epsilon-related transcript. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):6944–6948. doi: 10.1073/pnas.90.15.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wange R. L., Malek S. N., Desiderio S., Samelson L. E. Tandem SH2 domains of ZAP-70 bind to T cell antigen receptor zeta and CD3 epsilon from activated Jurkat T cells. J Biol Chem. 1993 Sep 15;268(26):19797–19801. [PubMed] [Google Scholar]
- Weiss A. T cell antigen receptor signal transduction: a tale of tails and cytoplasmic protein-tyrosine kinases. Cell. 1993 Apr 23;73(2):209–212. doi: 10.1016/0092-8674(93)90221-b. [DOI] [PubMed] [Google Scholar]
- Witthuhn B. A., Quelle F. W., Silvennoinen O., Yi T., Tang B., Miura O., Ihle J. N. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993 Jul 30;74(2):227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]