Figure 1.
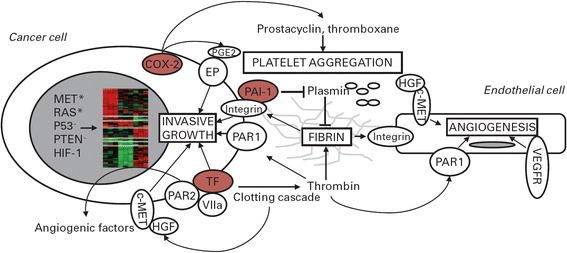
Hemostasis genes promote tumor progression. Activated oncogenes (MET*, RAS*), hypoxia-inducible factor-1 (HIF-1), and loss of tumor suppressor genes (PTEN-, P53-) induce transcriptional programs (nuclear heatmap) including tissue factor (TF), cyclo-oxygenase 2 (COX-2), and plasminogen-activator inhibitor 1 (PAI-1) upregulation. These, in turn, promote hemostasis activation and fibrin deposition. Fibrin forms a provisional matrix that favors angiogenesis and supports integrin-mediated cell adhesion and migration. Coagulation proteases activate hepatocyte growth factor (HGF), and thus the receptor encoded by the MET proto-oncogene (c-MET), which is expressed by endothelial and cancer cells. TF and thrombin generated by the coagulation cascade activate cell surface receptors (protease-activated receptors [PAR]-1 and −2). COX-2 catalyzes the synthesis of prostacyclin and thromboxane, which modulate platelet aggregation, and prostaglandin E2 (PGE2). The latter binds cell surface E-series prostaglandin receptors (EP). Besides inhibiting plasmin and fibrin degradation, PAI-1 promotes integrin recycling. MET, TF, PARs, EP, vascular endothelial growth factor receptor (VEGFR), and integrins cooperate in regulating cancer cell invasive growth and angiogenesis [23]
