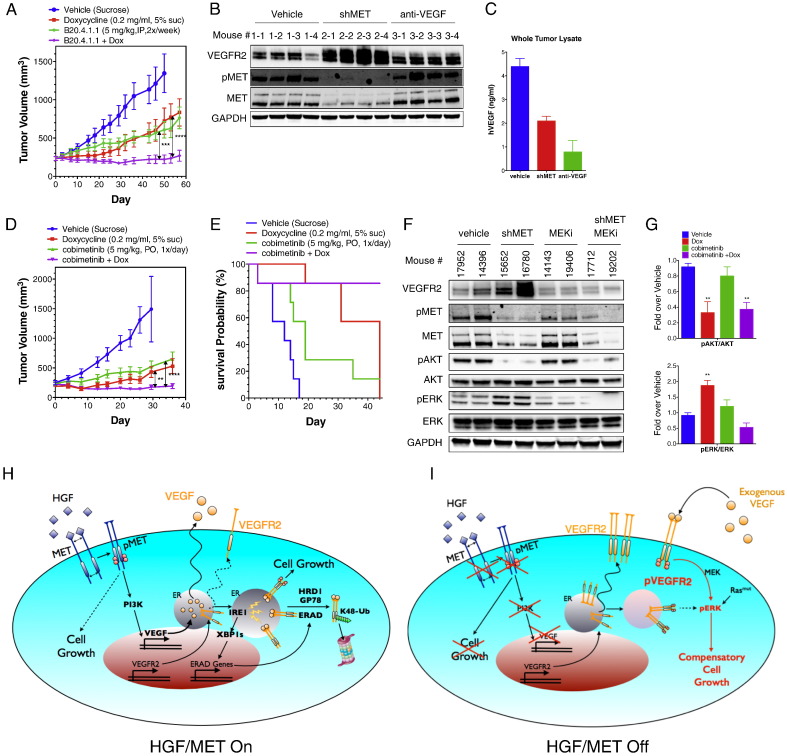
Fig. 7.
Combined inhibition of MET and VEGF blocks tumor growth.
(A) Effect of MET knockdown, VEGF neutralization, or both treatments on in vivo tumor growth. H441 shMET cells were injected subcutaneously into mice and tumors were allowed to grow and reach a volume of ~ 200 mm2. Mice were then randomized into 4 groups and treated i.p. with vehicle (sucrose), Dox (0.2 mg/ml), anti-VEGF neutralizing antibody (B20-4.1.1, dosed at 5 mg/kg, twice per week), or Dox plus anti-VEGF antibody and tumor volume was monitored for the indicated time period. Error bars indicate S.E.M., ***p < 0.005; ****p < 0.001 for indicated comparison.
(B and C) End-of-study tumor samples (n = 4 per group) were analyzed by immunoblot for VEGFR2, phospho-MET and MET protein (B) or by VEGF ELISA (C).
(D) Effect of MET knockdown, MEK inhibition, or both treatments on tumor growth. H441 tumors were established as in A. Mice were randomized into 4 groups and treated with vehicle (sucrose), Dox (0.2 mg/ml), MEK SMI (cobimetinib, dosed p.o. at 5 mg/kg once per day), or Dox plus MEK SMI and tumor volume was monitored for the indicated time period. Error bars indicate S.E.M., **p < 0.01; ****p < 0.001 for indicated comparison.
(E) Survival probability was calculated based on 2 times the initial tumor size as the threshold predicting death.
(F) End-of-study tumor samples were analyzed by immunoblot for VEGFR2, phospho-MET, MET, phospho-AKT, AKT, phospho-ERK and ERK protein.
(G) Densitometric analysis of phospho-AKT/AKT or phospho-ERK/ERK for end-of-study tumor samples. Error bars indicate S.E.M., n = 5 per group; **p < 0.01 for indicated comparison.
(H and I) Model depicting cellular events in the context of HGF–MET activation or its disruption. (H) HGF–MET signaling induces VEGF production via the PI3K pathway. Newly synthesized VEGF and VEGFR2 proteins are folded in the ER. Premature interaction between VEGF and VEGFR2 within the ER activates IRE1α, leading to XBP-1 splicing and upregulation of ERAD genes. The ERAD-associated E3 ligases, HRD1 and gp78, mediate K48-linked ubiquitination of VEGFR2, leading to its proteasomal degradation. HGF–MET signaling is the main epithelial growth driver in this context. (I) MET disruption prevents PI3K-mediated VEGF induction. VEGFR2 is no longer directed to ERAD-mediated destruction and its upregulation leads to autophosphorylation as well as enhanced sensitivity to exogenous VEGF. While MET disruption blocks MET-stimulated growth, enhanced VEGFR2 signaling now drives compensatory proliferation. In tumors expressing both MET and VEGFR2 in the epithelial compartment, this compensatory growth limits the efficacy of MET-disruptive therapy. However, combined inhibition of both the HGF–MET and VEGF–VEGFR2 pathways overcomes this limitation and prevents tumor growth.