Fig. 1.
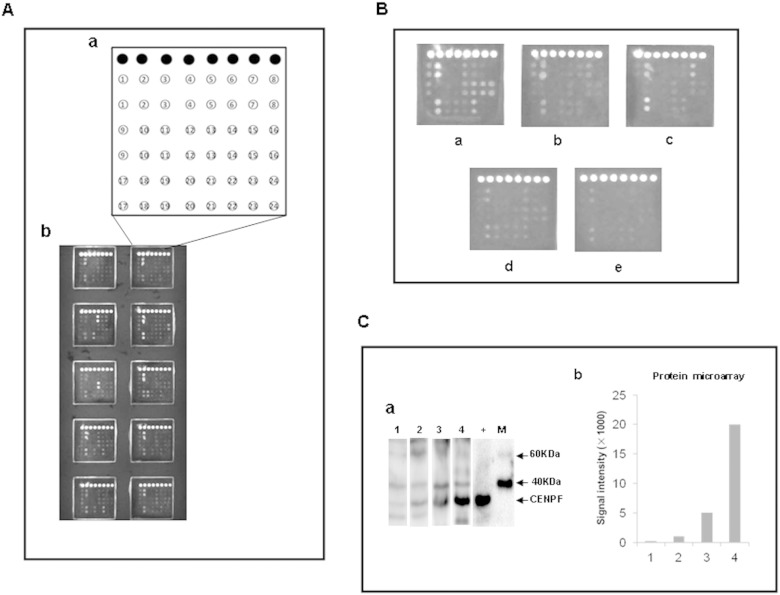
Microarray detection and validation of autoantibodies in liver cirrhosis, chronic hepatitis, HCC, other cancers and normal serum. A. Schematic representation of the protein microarray for high-throughput clinical validation. (a) Design of the protein microarray. ● IgG, 1. IGKC, 2. HSP60, 3. A1AT, 4. IMP-2, 5. FIBB, 6. HSPA6, 7. ATPB, 8. Bovine serum albumin, 9. ALDH1A1, 10. PDIA1, 11. ENO1, 12. ANXA4, 13. CENPF, 14. SBP1, 15. ACY1, 16. hnRNP A2, 17. K2C1, 18. AIF, 19. CRT, 20. RGN, 21. PRDX3, 22. HINT1, 23. TBB4B, 24. Sample liquid (0.01 M PBS, pH 7.0); (b) scan images of a representative antigen array. B. Microarray detection of serum sample. Individual arrays were incubated with HCC serum (a), liver cirrhosis serum (b), chronic hepatitis serum (c), serum from other cancers (d), or normal serum (e). C. Western Blots showing a pattern of antibody titers to CENPF in HCC sera detected by the protein microarray. (a) Representative Western Blot with recombinant proteins CENPF showing reactivity of HCC sera with various levels of auto-antibody to CENPF detected by the protein microarray. The lane with “+” indicates antibody against GST used as a positive control; lane 1, a normal human serum used as negative control; lanes 2–4, three HCC positive sera 569, 462 and 635, respectively with signal intensities of 1025, 5068 and 20018 detected by the protein microarray; M. EasySee Western Marker (TransGen Biotech, Beijing). (b) Signal intensity of autoantibody against CENPF in HCC sera detected by the protein microarray. Lane 1: the normal serum sample; lanes 2–4: the three HCC positive sera 569, 462 and 635.