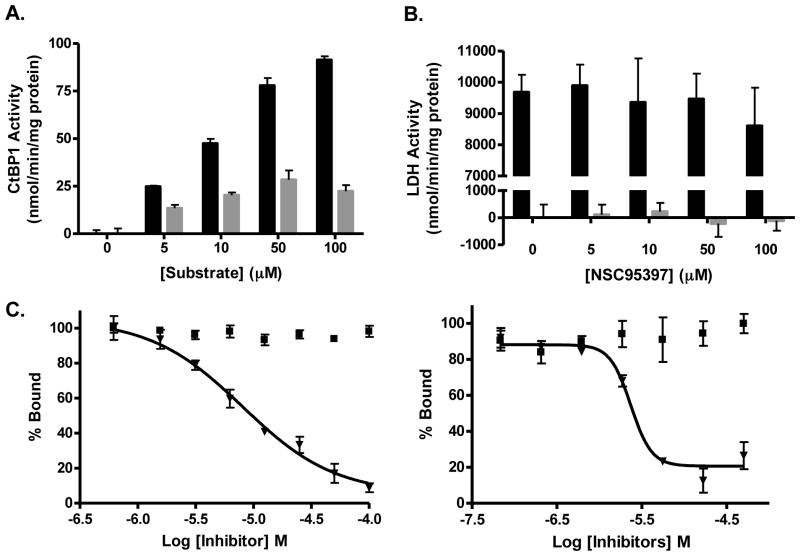
Figure 4. NSC95397 is a weak substrate for CtBP1 and does not inhibit other cellular dehydrogenases.
(A) CtBP1 enzymatic activity (nmol NADH/min/mg protein) was determined spectrophotometrically in the presence of 150 μM NADH and the indicated concentrations of NSC95397 (
 ) and MTOB (■). No activity was observed in the absence of substrate. (B) NSC95397 is unable to inhibit another NADH dependent enzyme, LDH. NSC95397 did not significantly inhibit enzymatic activity in the presence of 5 μM pyruvate (■) nor did it exhibit significant consumption of NADH without pyruvate (
) and MTOB (■). No activity was observed in the absence of substrate. (B) NSC95397 is unable to inhibit another NADH dependent enzyme, LDH. NSC95397 did not significantly inhibit enzymatic activity in the presence of 5 μM pyruvate (■) nor did it exhibit significant consumption of NADH without pyruvate (
 ). (C) The effect of a known substrate, MTOB, on the CtBP1-E1A interaction was monitored using both the AlphaScreen and fluorescence polarization assays. NSC95397 (▼) was able to disrupt the interaction, while MTOB (■), the more efficient CtBP1 substrate, was ineffective at disrupting the interaction.
). (C) The effect of a known substrate, MTOB, on the CtBP1-E1A interaction was monitored using both the AlphaScreen and fluorescence polarization assays. NSC95397 (▼) was able to disrupt the interaction, while MTOB (■), the more efficient CtBP1 substrate, was ineffective at disrupting the interaction.