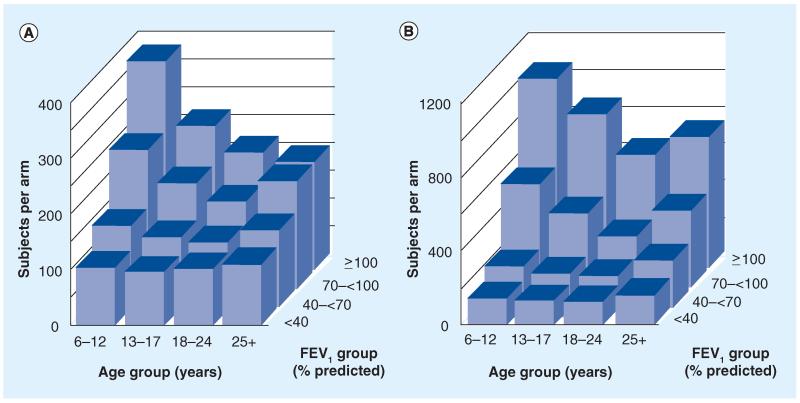
Figure 2. Sample size requirements for 1:1 randomized exacerbation risk studies conducted in different age and FEV1 subgroups.
Sample sizes per study arm required to detect a 40% reduction (hazard ratio: 0.6) in treatment for exacerbation with (A) inhaled antibiotics, oral fluoroquinolones or intravenous antibiotics and with (B) intravenous antibiotics. Values are provided for subgroups divided into age (abscissa axis) and FEV1% predicted (z-axis) subgroups. Estimates assume a 6-month blinded study, 1:1 randomization, 80% power and α = 0.05. Note that the ordinate scale in (B) is three-times the size of that of (A) [66].