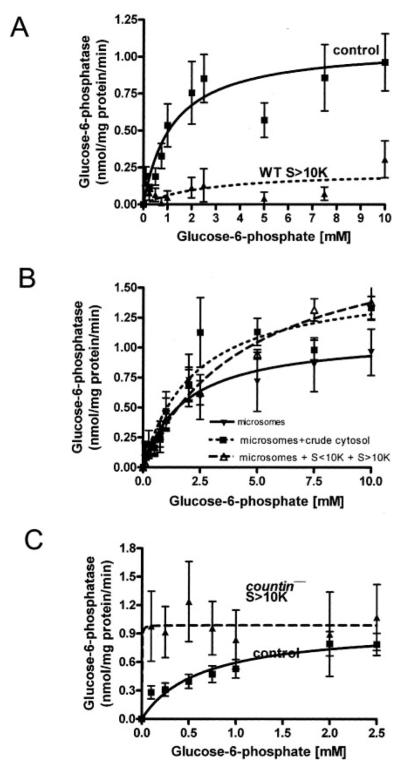
FIGURE 3. The effect of different cell fractions on the glucose-6-phosphatase activity of wild-type crude microsomes.
WT and countin− cells were starved by shaking in PBM, harvested at 6 h of starvation, and lysed by freezing/thawing. Cell lysates were then clarified by centrifugation, and the clarified lysates were fractionated by ultracentrifugation. The supernatants from the ultracentrifugation were further fractionated by using 10-kDa cutoff spin filters. Wild-type crude microsomes were resuspended in S>10K, crude cytosol, or reconstituted crude cytosol from wild-type and countin− cells, and the activities of glucose-6-phosphatase were measured. The lines show Michaelis-Menten equations fit to the data using nonlinear regression. A, glucose-6-phosphatase activities were measured in crude microsomes resuspended in PB (control) or wild-type S>10K. Values are the means ± S.E. from six independent assays. B, glucose-6-phosphatase activities were measured in crude microsomes from wild-type cells resuspended in PB (microsomes), WT crude cytosol (microsomes + crude cytosol), or reconstituted WT crude cytosol (microsomes + S<10K + S>10K). Values are the means ± S.E. from seven independent assays. C, glucose-6-phosphatase activities were measured in WT crude microsomes resuspended in PB (control) or S>10K from countin− cells. The presence of S>10K from countin− cells significantly increased the glucose-6-phosphatase activity at the physiological level of glucose 6-phosphate in wild-type cells (~114 μm) (paired t test, p < 0.05). Values are means ± S.E. from 10 independent assays.