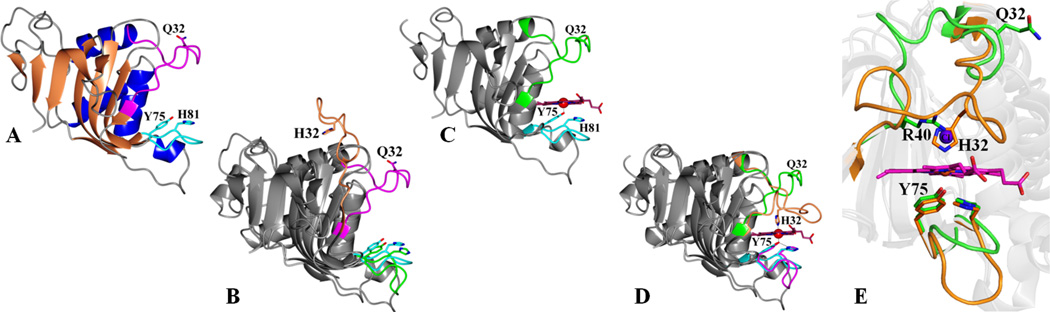
Figure 2.
(A) Structure of apo-HasAyp tet with the Q32 loop shown in magenta and the Y75 loop in cyan; Q32, Y75 and H81 are shown in sticks. (B) Superimposed structures of apo-HasAp (PDB ID: 3MOK) and apo-HasAyp tet where the H32 loop in apo-HasAp is shown in coral and the Q32 loop in apo-HasAyp tet in magenta. (C) The structure of holo-HasAyp is very similar to that of apo-HasAyp; the Q32 loop is in green and the Y75 loop in cyan. (D) Superposition of holo-HasAyp and holo-HasAp (PDB ID: 3ELL) structures. The hemin iron in HasAp is coordinated by Y75 and H32, whereas in holo-HasAyp it is coordinated by Y75 (also see Figure 3). (E) Zoomed-in view comparing the heme-binding pockets of holo-HasAyp (green) and holo-HasAp (orange); a chloride ion (purple sphere) in the distal pocket of HasAyp is in the position occupied by the side chain of H32 in holo-HasAp and holo-HasAsm. Structures were superimposed using the program Superpose (16).