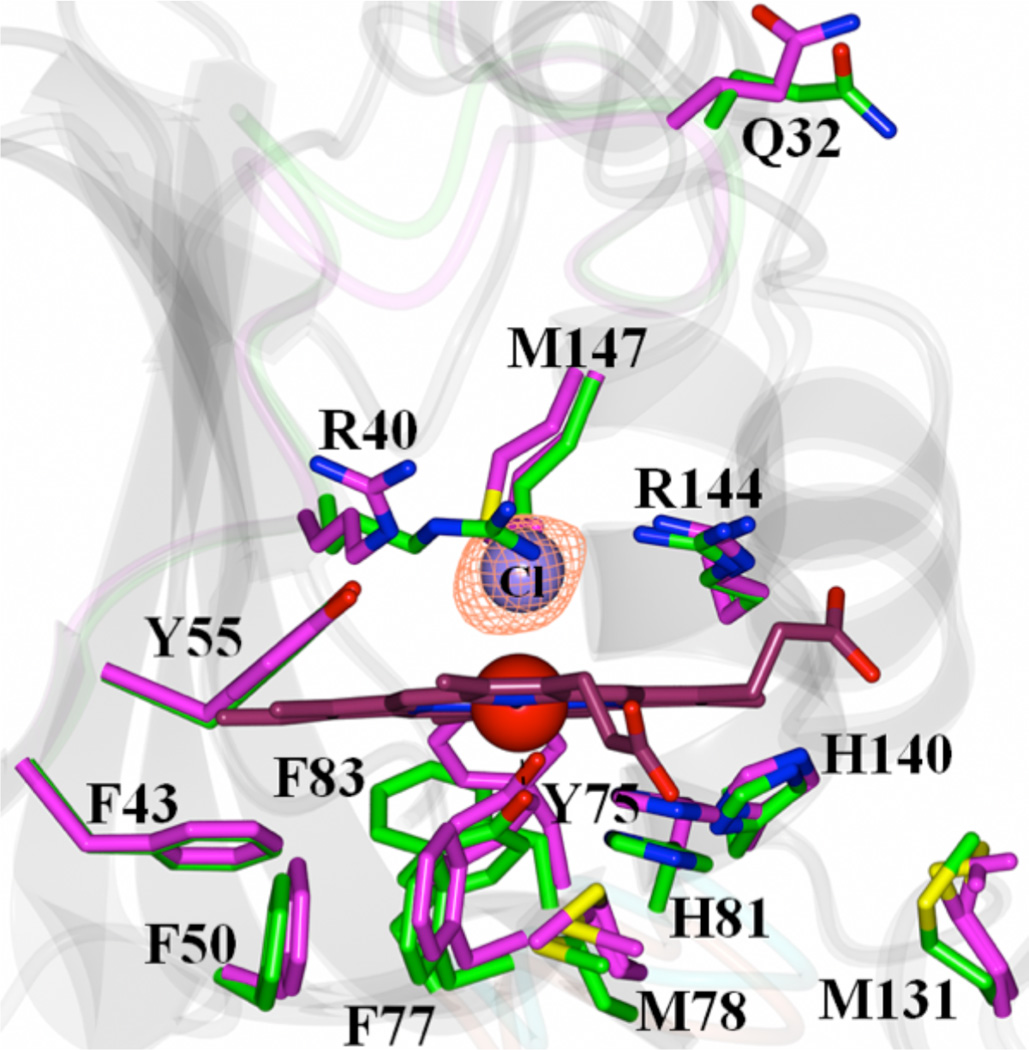
Figure 3.
View of the heme-binding pocket in the superimposed structures of apo-HasAyp tet and holo-HasAyp illustrate the minor structural differences between apo-(magenta) and holo-(green) HasAyp. The distal site in the holo protein is coordinated by a chloride ion; the Fo-Fc omit map contoured at 3 σ is shown in mesh representation.