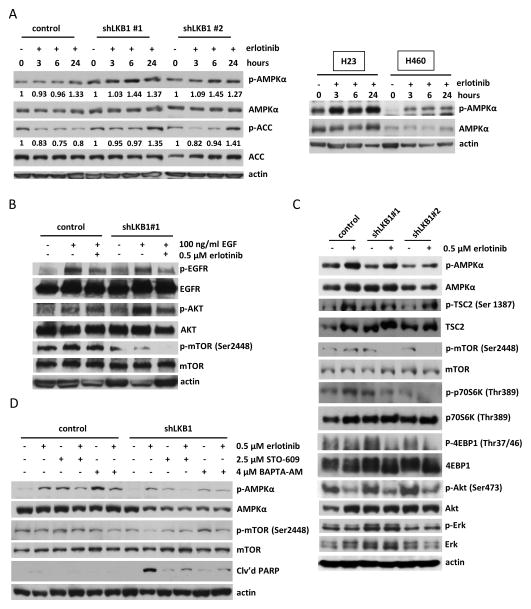
Figure 5. Erlotinib-induced activation of AMPKα selectively inhibits mTOR signaling pathway in LKB1-deficient cells.
(a) Left panel: Erlotinib activated AMPKα and inhibited ACC in shLKB1 Calu-6 cells. Following treatment with 0.5 μM erlotinib for the indicated time, cell lysates were analyzed by Western blotting. The expression of phosphorylated AMPKα was calculated as the ratio of phosphorylated AMPKα to total AMPKα protein expression using densitometric analysis. The ratio of phosphorylated to total ACC was calculated similarly. All expression ratios were normalized to the untreated group. Right panel: Erlotinib also induced phosphorylated AMPKα in LKB1 mutant H23 and H460 cells. (b) Erlotinib inhibited EGF-stimulated EGFR phosphorylation and mTOR phosphorylation in shLKB1 Calu-6 cells. Cells were treated with 0.5 μM erlotinib for 72 h. After serum starvation for 24 h, cells were stimulated by 100 ng/ml EGF for 10 minutes and cell lysates were then analyzed by Western blotting. (c) Erlotinib blocked mTOR pathway signaling and activated AMPKα in shLKB1 Calu-6 cells. After 24 h of 0.5 μM erlotinib treatment, cell lysates were analyzed by Western blotting using specific antibodies for the indicated proteins. Blockade of mTOR activity following erlotinib treatment resulted in decreased phosphorylation of p70S6K and p-4EBP1 in shLKB1 Calu-6 cells. (d) Erlotinib-induced pAMPKα phosphorylation was reduced by addition of STO-609 (a CAMKKβ inhibitor) or BAPTA-AM (an intracellular calcium chelator) in shLKB1 Calu-6 cells. Cells were co-treated with 0.5 μM erlotinib and 2.5 μM STO-609 or 4 μM BAPTA-AM for 24 h and cell lysates were analyzed by Western blotting. The blots are representative of three independent experiments.