Table 1.
Small Molecule Plk1 Inhibitor Structures, Mechanisms of Action, and Preclinical Activities
| Agent/Structure | Mechanism of Action | IC50 |
|---|---|---|
Rigosertib (ON 01910.Na) (benzylstyryl sulphone)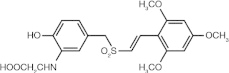
|
Affects microtubule dynamics | Plk1 = 9-10 nM 10- to 20-fold higher concentrations were required for inhibition of Plk2 [56] |
Volasertib (BI 6727) (dihydropteridinone derivative)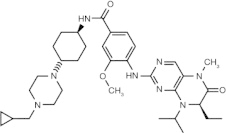
|
ATP-competitive inhibitor | Plk1 = 0.87 nM Plk2 = 5 nM Plk3 = 56 nM [57] |
GSK 461364 (thiophene derivative)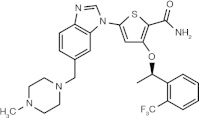
|
ATP-competitive inhibitor | Plk1 ≤ 0.5 nM⁎ Plk2 = 860 nM⁎ Plk3 = 1000 nM⁎[58] |
GW843682 (benzimidazole thiophene)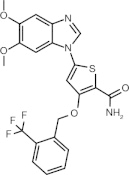
|
ATP-competitive inhibitor | Plk1 = 2.2 nM Plk2 = N/A Plk3 = 9.1 nM [59] |
PLHS-Pmab ((2S,3R)-2-amino-3-methyl-4-phosphonobutyric acid)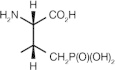
|
Interferes with Plk1 PBD functions in vitro and in vivo | Not reported [60] |
PPG (benzotropolone-containing compound)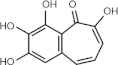
|
Inhibits PBD-dependent binding in vitro and in vivo | Plk1 = ~ 0.3 μM (in glutathione S-transferase pull-down assay) Plk2 = N/A Plk3 = N/A [61] |
Poloxin (thymoquinone derivative)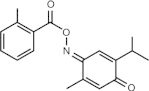
|
Interferes with Plk1 PBD functions in vitro and in vivo | Plk1 = 4.8 μM Plk2 = 18.7 μM Plk3 = 53.9 μM [62] |
N/A, not applicable.
IC50 values for GSK461364A were determined on the basis of the intrinsic binding constant (Ki⁎app), which was calculated by applying the Cheng-Prusoff relationship for a competitive inhibitor (ATP Kmapp = 16 μM) to the IC50 value obtained following a 60-minute preincubation in the presence of GSK461364A [58].
