Abstract
The objective of this study was to optimize the extraction conditions of phenolic and flavonoids compounds from quinoa (Chenopodium quinoa) seeds using ultrasound assistance technology. A randomized central composite face-centered design was used to evaluate the effect of extraction temperature, ethanol concentration in the solvent, and ultrasound power on the total phenolic content (TPC), total flavonoid content (TFC) and antioxidant activity by response surface analysis. Predicted model equations were obtained to describe the experimental data regarding TPC, TFC and antioxidant activity, with significant variation in the linear, quadratic, and interaction effects of the independent variables. Regression analysis showed that more than 88 % of the variability was explained by the models. The best extraction conditions obtained by simultaneous maximization of the responses were: extraction temperature of 60 °C, 80 % ethanol as solvent and non-application of ultrasounds. Under the optimal conditions, the corresponding predicted response values were 103.6 mg GAE/100 g dry weight (dw), 25.0 mg quercetin equiv./100 g dw and 28.6 % DPPH radical scavenging, for TPC, TFC and antioxidant activity, respectively. The experimental values agreed with those predicted within a 95 % confidence level, indicating the suitability of the employed model. HPLC analysis of the obtained extracts confirmed the highest phenolic compound yield in the extract obtained under optimal extraction conditions. Considering the characteristics of the antioxidant-rich extracts obtained, they could be consider for potential application in the food industry, as nutraceutical and functional foods ingredient or well as replacement of synthetic antioxidants.
Keywords: Chenopodium quinoa, Ultrasound assisted extraction, Phenolic compounds, Flavonoids, Antioxidant activity
Introduction
Quinoa (Chenopodium quinoa) is a native food plant traditionally cultivated in the Andean region of South America. Although a lesser-known plant, there has been growing interest due to its nutritional value but also due to the strong tolerance to stressing abiotic conditions (Vega-Gálvez et al. 2010). Quinoa’s aptitude to produce high-protein grains under environmental extreme conditions makes it important for the diversification of future agricultural systems (Bhargava et al. 2006). While most quinoa is still cultived in South America, it has been introduced in Europe, North America, Asia and Africa with high yields (Abugoch James 2009).
Nutritionally, the grain provides high protein content and a better-balanced amino acid composition than the traditional cereals. Also, it is a good source of dietary fibre and unsaturated fats (Alvarez-Jubete et al. 2010). In addition it contain adequate levels of vitamins and minerals (Konishi et al. 2004). But, beyond their basic nutritional function of supplying nutrients, different authors have reported that quinoa seeds represent a potential rich source of phenolic compounds, particularly flavonoids, with health-promoting and/or disease-preventing properties (Abugoch James 2009; Alvarez-Jubete et al. 2010; Hirose et al. 2010; Repo-Carrasco-Valencia et al. 2010).
Phenolic compounds are secondary plant metabolites which can prevent several degenerative diseases dependent of oxidative stress, through antioxidant action and/or the modulation of several protein functions (Hirose et al. 2010; Rice-Evans et al. 1997). The optimum extraction of phenolic compounds is therefore an important step prior to eventual purification and application of the extracts. Considering the diversity of natural sources of polyphenols, as well as the structure and physicochemical properties of these compounds, specific processes should be designed and optimized for each phenolic source (Silva et al. 2007).
Despite of the interesting properties of quinoa seeds, to the best of our knowledge, the optimal conditions for the extraction of phenolic compounds have not been well investigated.
The extraction of phenolic compounds from plant materials can be carried out in a variety of ways, using conventional extraction processes (e.g. maceration, infusion and Soxhlet extraction) or more recent technologies (e.g. ultrasound-assisted extraction, microwave-assisted extraction and supercritical fluid extraction). Many factors, such as time of extraction, solid-liquid ratio, temperature, particle size and solvent composition, among others, may significantly influence the efficacy of solid-liquid extraction (Liyana-Pathirana and Shahidi 2005). The polarities of phenolic compounds range from polar to non-polar, thus a wide range of solvents such as hexane, ethyl acetate, methanol, ethanol, acetone, water and their mixtures have been used for the extraction of these compounds (Liyana-Pathirana and Shahidi 2005; Madhujith and Shahidi 2006). However, the use of toxic solvents should be avoided to reduce health and environmental risks (Durling et al. 2007). Particularly, water-ethanol mixtures are interesting for the extraction of natural antioxidants, since water and ethanol may be used as food grade solvents and their mixtures have shown to be more efficient for phenolic compound extraction than the pure solvents (Liyana-Pathirana and Shahidi 2005).
Application of ultrasonic-assisted extraction (UAE) has been mentioned as a potential technology in the extraction of phenolic compounds from plant materials, since it is an economical alternative with few instrumental requirements and adaptable on a small or large scale (Ma et al. 2008; Galvan d’Alessandro et al. 2012). In addition, compared to traditional extraction methods, UAE offers many advantages, such as higher extraction yields, shorter extraction time and low solvent volumes (Ma et al. 2008). To our knowledge, there are no data on the optimization of UAE of phenolic compounds from C. quinoa seeds.
The purpose of this study was to optimize the experimental conditions to obtain antioxidant-rich natural extracts from quinoa seeds using food grade solvents and ultrasound assistance, as well as to identify the phenolic profiles of quinoa extracts. To optimize the extraction, the effect of ultrasound power, temperature and solvent composition on yields of total phenolic content (TPC), total flavonoid content (TFC) and antioxidant activity was studied.
Materials and methods
Plant material
Quinoa seeds (Chenopodium quinoa Willd., var. Real) were obtained from Buenos Aires province, Argentina, during September 2012. Seeds were cleaned and stored in polyethylene containers at room temperature until use. Before extraction, the seeds were milled using a laboratory grinder (Yellow line, A10, IKA-Werke, Staufen, Germany) and sieved (<0.5 mm particle size). The fine powder was packed and stored at room temperature in a dry and dark place until use.
Chemicals
Folin-Ciocalteau reagent, 2,2-diphenyl-1-picrylhydrazyl (DPPH), formic acid, gallic acid, p-hydroxybenzoic acid, vanillic acid, p-coumaric acid, ferulic acid, quercetin and kaempferol were supplied by Sigma-Aldrich Chemical Co. (St. Louis, USA). Sodium carbonate, sodium nitrite, aluminum chloride, sodium hydroxide, ethanol and methanol (HPLC grade) were supplied by Merck (Darmstadt, Germany).
Experimental equipment
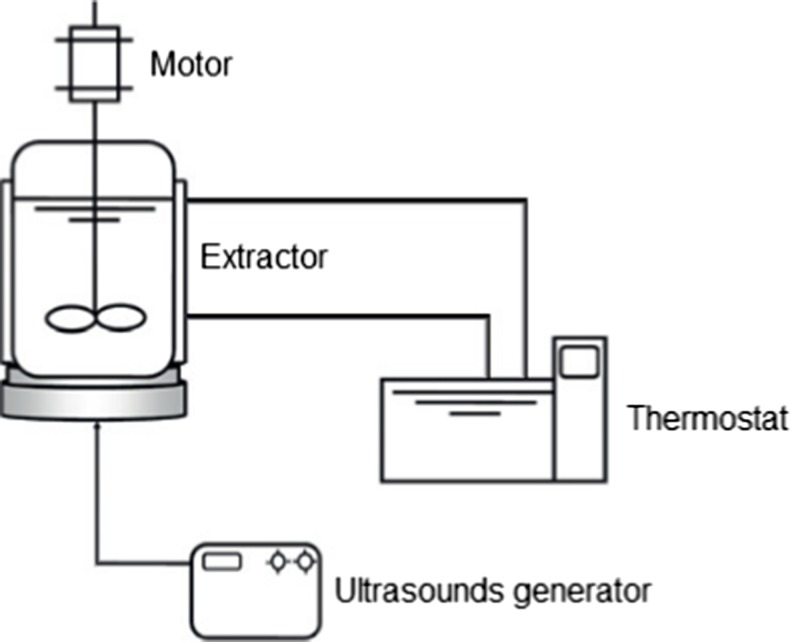
The extraction of phenolic compounds from quinoa seeds was performed in a glass contactor of 1 L, equipped of a generator of ultrasounds (Nexus P198-R, SinapTec, Lezennes, France) and agitation (Fig. 1). The temperature of extraction was maintained constant using an external circulating water bath connected to a thermostat. The experiments were carried out by varying the following extraction parameters: temperature of extraction (20, 40 and 60 (°C)), ethanol content (0, 40 and 80 (%, v/v)), and ultrasound power (0, 50 and 100 (W)). In the case of ultrasound assistance, the sonication was applied in continuous mode at frequency of 30.8 kHz and the maximal power input density was 250 W/L. In such runs, samples (20 g) were suspended in the solvent media (400 mL) and then submitted to different extractions conditions for 60 min. Solid-solvent ratio (1:20) and extraction time were optimized in preliminary experiments using one-factor-at-a-time approach (data not shown). Then, the extracts were centrifuged for 10 min at 10,000 rpm (Eppendorf Centrifuge 5804 R, Hamburg, Germany) and the supernatants were carefully removed for further analysis.
Fig. 1.
Schematic representation of experimental equipment for ultrasound assisted extraction
Experimental design and statistical analysis
The optimization of phenolic compounds extraction from quinoa seeds was carried out using three independent process variables through a 23 factorial experimental design with six star points and four replicates at the center point, according to central composite face-centered design (CCFD). The experimental design conditions used in this work are shown in Table 1.
Table 1.
Experimental design conditions and yields of total phenolic content (TPC), total flavonoid content (TFC), antioxidant activity, and HPLC total phenolics from quinoa seeds extracts
| Run | Temperature (°C) | Ethanol concentration (%) | Ultrasound power (W) | TPC (mg GAE/100 g dw) | TFC (mg QE/100 g dw) | DPPH radical scavenging (%) | HPLC total phenolics (mg/100 g dw) |
|---|---|---|---|---|---|---|---|
| 1 | 20 (−1) | 0 (−1) | 0 (−1) | 67.50 | 1.65 | 14.2 | 2.1 |
| 2 | 60 (+1) | 0 (−1) | 0 (−1) | 73.47 | 2.69 | 15.9 | 2.3 |
| 3 | 20 (−1) | 80 (+1) | 0 (−1) | 96.79 | 16.85 | 27.4 | 21.5 |
| 4 | 60 (+1) | 80 (+1) | 0 (−1) | 102.86 | 26.93 | 28.9 | 22.6 |
| 5 | 20 (−1) | 0 (−1) | 100 (+1) | 70.01 | 2.73 | 11.8 | 2.6 |
| 6 | 60 (+1) | 0 (−1) | 100 (+1) | 75.36 | 3.17 | 12.4 | 2.9 |
| 7 | 20 (−1) | 80 (+1) | 100 (+1) | 77.68 | 15.97 | 27.0 | 21.2 |
| 8 | 60 (+1) | 80 (+1) | 100 (+1) | 91.61 | 16.93 | 27.7 | 21.7 |
| 9 | 40 (0) | 40 (0) | 50 (0) | 76.79 | 7.41 | 21.1 | 16.6 |
| 10 | 40 (0) | 40 (0) | 50 (0) | 77.86 | 7.93 | 20.0 | 15.7 |
| 11 | 40 (0) | 40 (0) | 50 (0) | 80.43 | 8.93 | 20.6 | 16.1 |
| 12 | 40 (0) | 40 (0) | 50 (0) | 78.25 | 10.01 | 20.8 | 15.4 |
| 13 | 20 (−1) | 40 (0) | 50 (0) | 85.36 | 8.81 | 19.3 | 16.4 |
| 14 | 60 (+1) | 40 (0) | 50 (0) | 92.68 | 11.41 | 21.1 | 15.0 |
| 15 | 40 (0) | 0 (−1) | 50 (0) | 74.29 | 2.29 | 12.7 | 2.8 |
| 16 | 40 (0) | 80 (+1) | 50 (0) | 88.21 | 18.77 | 27.1 | 20.1 |
| 17 | 40 (0) | 40 (0) | 0 (−1) | 79.64 | 12.97 | 21.6 | 17.7 |
| 18 | 40 (0) | 40 (0) | 100 (+1) | 81.55 | 12.57 | 20.8 | 16.0 |
The TPC, TFC, and % DPPH radical scavenging were determined as responses of the experimental design. Statistical analysis and response surface plots were performed using Design Expert program (8.0.7.1 version, Stat-Ease Inc., MN, USA). Data were analyzed using analysis of variance ANOVA with a confidence level of 95 %. The quadratic equation model used in the response surface analysis was as follows:
| 1 |
where Y is theresponse; b0 is the constant coefficient; b1, b2, and b3 are the linear coefficients of extraction temperature (X1), ethanol concentration (X2) and ultrasound power (X3), respectively; b11, b22 and b33 are the squared coefficients of X1,X2 and X3, respectively; b12, b13 and b23 are the interaction coefficients of X1,X2 and X3, respectively.
Determination of total phenolic content (TPC)
TPC in extracts was determined using Folin-Ciocalteau reagent (Singleton et al. 1998). The liquid extracts were diluted and mixed with Folin-Ciocalteau reagent (2 N) and 20 % sodium carbonate solution. The mixture was incubated in the dark for 2 h. After incubation, the absorbance of the mixture was measured at 765 nm using an UVmini 1240 spectrophotometer (Shimadzu, France). The results were expressed as equivalent of gallic acid (GAE) in mg per 100 g quinoa seeds in dry weight basis (dw).
Total flavonoid content (TFC)
TFC was determined by the aluminum chloride colorimetric method as described by Dini et al. (2010) with slight modifications. Briefly, 0.25 mL aliquot of the extract was mixed with 2 mL of distilled water and 0.15 mL of 5 % sodium nitrite solution in a test tube. After 5 min, 0.15 mL of 10 % aluminum chloride solution was added. At 6 min, 1 mL 1 M sodium hydroxide solution was added to the mixture. Immediately, the solution was diluted with 1.2 mL of distilled water and thoroughly mixed. Absorbance of the final mixture was determined at 510 nm against a blank reaction. Total flavonoid content of extracts was expressed as equivalent of quercetin (QE) in mg per 100 g quinoa seeds in dry weight basis.
Antioxidant activity
Antioxidant activity of quinoa seeds extracts was evaluated by DPPH radical scavenging activity measured according to Brand-Williams et al. (1995). Aliquots (50 μL) of extracts were added to 1,950 μL of a methanolic solution (40 μM) of DPPH radical. After agitation, the mixture was incubated in the dark for 30 min and the absorbance was measured at 517 nm. The antioxidant activity was expressed as percentage of DPPH radical scavenging calculated according to the following equation (Liyana-Pathirana and Shahidi 2005):
| 2 |
where AC(30) corresponds to absorbance of DPPH radical + methanol at t = 30 min and AS(30) to absorbance of DPPH radical + sample at t = 30 min.
Chromatographic analysis of phenolic acids and flavonoids
Reversed phase HPLC method for determination of phenolic acids and flavonoids was used in the conditions described in a previous work (Carciochi et al. 2014). The analytical HPLC system employed consisted of a Waters 600 high performance liquid chromatograph equipped with a Waters 2996 diode array detector (Waters Corporation, Milford, MA, USA). Software used for data acquisition and control of HPLC pumps, autosampler, and diode array system was Empower (Waters Corporation, Milford, MA, USA). The wavelengths used for identification and quantification of phenolic acids and flavonoids were 280 and 370 nm, respectively. The separation was carried out on a reversed phase Gemini C6 – Phenyl column (250 × 4.6 mm, 3 μm) (Phenomenex, Torrance, CA, USA). The mobile phase consisted of two solvents; 0.1 % formic acid aqueous solution (A) and methanol containing 0.1 % formic acid (B) operating in gradient form. The flow rate of the mobile phase was 0.6 mL/min and the injection volumes for all samples and standards were 20 μL. Phenolic acids and flavonoids were quantified as aglycones in duplicate using the external standard method and the amount of each compound was expressed as mg per 100 g of dry matter.
Results and discussion
Extraction optimization
Optimization of extraction conditions was conducted in 18 randomized runs to study the effect of different variables on the yields of TPC and TFC, as well as on the antioxidant activity of the extracts. The three independent variables, their coded and uncoded values and the results of evaluated responses are shown in Table 1. Phenolic compounds extracted from quinoa seeds ranged from 67.50 to 102.86 mg GAE/100 g sample and extracted flavonoids varied between 1.65 and 26.93 mg QE/100 g sample, showing considerable dependence of the yields from the extraction conditions, indicating the importance to optimize the extraction process. The software generated three regression equations (one for TPC, one for TFC and one for antioxidant activity of the extracts, respectively) showing the effects of each factor and their interactions on each evaluated response. Analysis of the regression models (ANOVA) is summarized in Table 2. The corresponding coefficients of determination (R2) of the models were 0.8864, 0.9549, and 0.9927 for TPC, TFC and antioxidant activity, respectively. These values showed that more than 88.64 % of the total variation in the response was explained by the models. Also, the very low p-values (<0.0001) in each evaluated response indicated the significance of the model terms. The non-significant value of lack of fit (more than 0.05) showed that the models could be used to predict the responses in this study (Hamsaveni et al. 2001). Regression models were obtained with the significant regression coefficients of each evaluated variable and their interactions at 95 % confidence level. Neglecting the non-significant terms the predictive equations were obtained (Table 3). To determine the optimal levels of the evaluated variables on extraction of phenolic compounds and antioxidant activity, response surface plots were established using the predictive equation of the fitted models.
Table 2.
Analysis of variance (ANOVA) for the fitted quadratic polynomial models for optimization of extraction parameters
| Source | TPC (R2 = 0.8864) | TFC (R2 = 0.9549) | DPPH (R2 = 0.9927) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DFa | SSb | MSc | F-value | p-value | DF | SS | MS | F-value | p-value | DF | SS | MS | F-value | p-value | |
| Model | 5 | 1,350.87 | 270.17 | 18.72 | < 0.0001 | 6 | 767.43 | 127.90 | 38.87 | < 0.0001 | 4 | 518.70 | 129.68 | 440.37 | < 0.0001 |
| Lack of Fit | 9 | 166.12 | 18.46 | 7.88 | 0.0582 | 8 | 32.24 | 4.03 | 3.05 | 0.1942 | 10 | 3.17 | 0.32 | 1.46 | 0.4196 |
| Pure Error | 3 | 7.02 | 2.34 | 3 | 3.96 | 1.32 | 3 | 0.65 | 0.22 | ||||||
a Degree of freedom
b Sum of squares
c Mean square
Table 3.
Predictive model equations of the experimental response variables
| Responses | Polynomial equations | |
|---|---|---|
| TPC (mg GAE/100 g dried seeds) | y = 79.63 + 3.87X 1 + 9.65X 2–2.41X 3–4.34X 2 X 3 + 3.70X 1 2 | (3) |
| TFC (mg QE/100 g dried seeds) | y = 9.44 + 1.51X 1 + 8.29X 2 + 1.20X 1 X 2 − 1.22X 1 X 3–1.56X 2 X 3 + 1.80X 3 2 | (4) |
| DPPH radical scavenging (%) | y = 20.58 + 0.63X 1 + 7.11X 2–0.83X 3 + 0.54X 2 X 3 | (5) |
Effect of extraction conditions on TPC and TFC
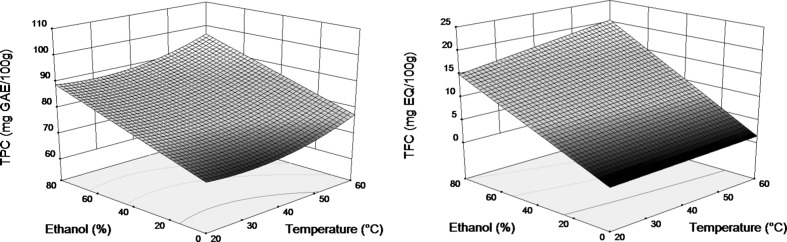
Varying water-ethanol ratios the solvent polarity, and consequently, the solubility of different phenolic compounds can be modified (Galvan d’Alessandro et al. 2012). In order to obtain highest TPC and TFC yields it is very important to find the best water-ethanol ratio. Figure 2 shows TPC and TFC as a function of ethanol content in the solvent and extraction temperature at fixed ultrasound power (50 W). It is shown that ethanol concentration had high influence with positive effect on yields of both responses. Indeed, solvent composition was the most significant parameter on total phenolics and flavonoids extraction. The TPC and TFC yields obtained with 40 % ethanol and especially with 80 % ethanol were higher than the obtained with water as solvent. The increase in ethanol concentration at fixed temperature led to a gradual increase in the TPC and TFC and reached a maximum in the region close to 80 % ethanol. It is worth mentioning that the positive effect of ethanol content in the solvent was much higher for flavonoids than for total phenolics.
Fig. 2.
Response surface plots showing interaction between ethanol concentration (X 2) and extraction temperature (X 1) at fixed ultrasound power (50 W) on TPC and TFC of quinoa seeds
It is well known that higher temperature improve the solubility but also the mass transfer due to increase of diffusion coefficients (Silva et al. 2007; Spigno and De Faveri 2007). Since it has been reported that some families of phenolic compounds can be denatured beyond a certain temperature value (Silva et al. 2007; Spigno and De Faveri 2007), 60 °C was chosen as the upper limit in the present study. The effect of temperature on TPC and TFC extraction seen in Fig. 2 is positive but clearly lower than the effect of solvent.
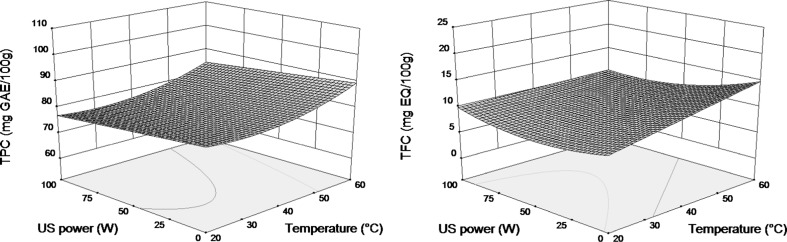
Figure 3 shows the effect of extraction temperature and ultrasound power on TPC and TFC yields at fixed ethanol concentration in the solvent (40 %). The yield of total phenolic compounds was almost constant between 20 and 40 °C, then it increased slightly with the increase of temperature reaching maximal values at the highest temperature tested (60 °C). When ultrasound assistance was not used it was observed that TFC yield increased gradually with the increase of temperature and a maximal TFC at 60 °C was reached. By other hand, when ultrasound assistance was applied, temperature effect on TFC values was very low.
Fig. 3.
Response surface plots showing interaction between extraction temperature (X 1) and ultrasound power (X 3) at fixed ethanol concentration (40 %) on TPC and TFC of quinoa seeds
Figure 3 shows also that ultrasound assistance did not improve TPC and TFC yields. Even a low negative effect of ultrasounds was observed at higher temperatures for both TPC and TFC yields. The low effect of ultrasound assistance in present study could be attributed to the pretreatment used (milling) and the possible thermo degradation of the target molecules. Indeed, the positive effect of ultrasound assistance has been reported to increase with the increase of particle size (Galvan d’Alessandro et al. 2012) and the particles used in present study were very fine (d < 0.5 mm). Also, some studies have reported that the amount of phenolic compounds decreased after ultrasound application, mainly due to a long period of ultrasound application to the same matrix (Da Porto et al. 2013; Pingret et al. 2013), temperatures higher than 40 °C (Carrera et al. 2012) or a combination of time (>20 min) and temperature (40 °C) on the extraction (Ma et al. 2008). Taking into account the obtained results in the present study, it is important to conclude that ultrasound assistance is not always appropriate to enhance the extraction yields and its efficiency is strongly related to the nature of the vegetal matrix, the type of compounds to be extracted and the pretreatment of the vegetal source. It should be mentioned that a significant increase of the extraction yields due to ultrasound assistance has been reported in a study where very similar extraction conditions (the same extraction device, ultrasound power, temperature range, and solvent composition), but a different matrix of phenolic antioxidants were used (Galvan d’Alessandro et al. 2012).
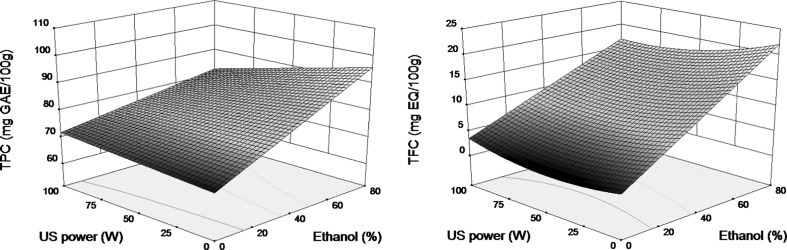
The observed low negative effect of ultrasounds on TPC and TFC was confirmed in Fig. 4 for various solvent compositions and temperature fixed at 40 °C. The clear positive effect of ethanol content in the solvent was also confirmed especially for flavonoids. This effect resulted lower for TPC yields in the case of ultrasound assistance.
Fig. 4.
Response surface plots showing interaction between ethanol concentration (X 2) and ultrasound power (X 3) at fixed extraction temperature (40 °C) on TPC and TFC of quinoa seeds
According to the surface graphs presented in Figs. 2, 3 and 4, extraction of phenolic compounds from milled quinoa seeds was higher at high extraction temperature, high ethanol content and when no ultrasound was applied.
As can be observed in Table 3, the significance of the polynomial coefficients (Eq. 3) confirmed that the most significant parameter on TPC yields was ethanol content in the solvent system (X2), followed by the interaction between ethanol concentration and ultrasound power (X2X3) and extraction temperature (X1). However, it was observed that quadratic effect of temperature (X12) and ultrasound power (X3) were also significant at 95 % confidence level, but with lower impact on extraction of total phenolic compounds. For TFC yields (Eq. 4), ethanol concentration (X2) was also the most significant variable. The parameters X1, X1X2, X1X3, X2X3 and X32 were also significant at 95 % confidence level, but with very low impact.
Effect of extraction conditions on antioxidant activity of the extracts
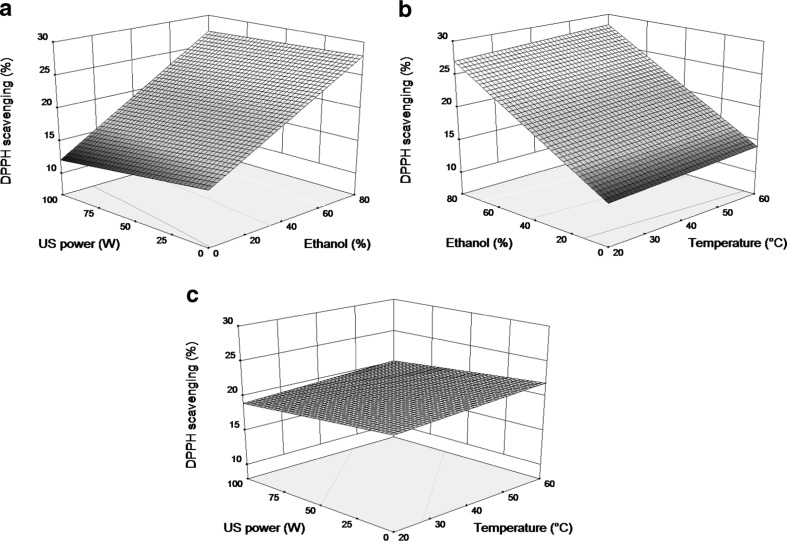
Most of the beneficial characteristics of phenolic compounds have been attributed to their antioxidant activity (Rice-Evans et al. 1997). Antioxidant phenolics present in vegetal samples may act as free radical scavengers, reducing agents, and potential chelators of metal ions, contributing to reduce the oxidative stress (Madhujith and Shahidi 2006). The DPPH method is one of the most extensively used antioxidant assays because it is a quick, reliable and reproducible method that can be used for examining the general antioxidant activity of natural substances as well as plant extracts in vitro (Koleva et al. 2002). DPPH radical scavenging assay was therefore used to monitor the capacity of the extracted compounds to scavenge free radicals in hydrophilic system. The effects of extraction temperature, solvent composition and ultrasound power and their interactions on the DPPH radical scavenging activity of quinoa seeds extracts were shown in Fig. 5. According to Eq. 5, ethanol content in the solvent system (X2) was the most significant parameter on antioxidant activity, while ultrasound power (X3), extraction temperature (X1) and the interaction between solvent composition and ultrasound power (X2X3) were also significant at 95 % confidence level, but with lower impact (Table 3). The antioxidant activity principally increased with the rise of ethanol concentration in the solvent system. The extraction temperature displayed a similar effect on the antioxidant capacity but with minor impact, while ultrasound power had a slight negative effect on the antioxidant capacity of extracts. The results obtained in the present study correlated also to the amounts of TPC and TFC presents in quinoa seed extract, which could be responsible for the antioxidant activity observed. On the basis of the obtained results one can conclude that the obtained extract of quinoa seeds with higher TPC and TFC showed also the highest antioxidant activity.
Fig. 5.
Response surface plots of quinoa seed extracts a showing interaction between extraction temperature and ethanol concentration, b showing interaction between ethanol concentration and ultrasound power, c showing interaction between extraction temperature and ultrasound power on antioxidant activity. The value of the missing variable in each plot was kept at the center point
Optimization of the extraction conditions
The main purpose of this study was to optimize the extraction process in order to maximize the extraction of antioxidant phenolic compounds from quinoa seeds. To optimize the process with two or more output responses, it is useful to use the concept of desirability function, available in the employed software. During optimization of extraction process, some of these responses need to be maximized, while others need to be minimized to obtain acceptable quality extracts. Desirability ranges from zero to one for any given response. A value of one represents the ideal case, while zero indicates that one or more responses are outside of the desirable limits. So, desirability function was developed with the following criteria: maximum TPC and TFC, and maximum antioxidant activity in the quinoa seed extracts. By applying desirability function, the optimum extraction conditions were obtained: temperature of 60 °C, ethanol concentration of 80 % and non-ultrasound assistance, which corresponded to one of the conditions used to build the model (run 4, Table 1). Under these extraction conditions TPC yield was 102.86 mg GAE/ 100 g dried seeds, TFC yield was 26.93 mg QE/100 g dried seeds and antioxidant activity was 28.9 measured as % DPPH radical scavenging. The corresponding predicted response values were 103.6 mg GAE/100 g dw, 25.01 mg QE/100 g dw and 28.6 % DPPH radical scavenging, for TPC, TFC and antioxidant activity, respectively. The experimental values agreed with those predicted within a 95 % confidence level.
Chromatographic analysis of the extracted phenolic compounds
HPLC-DAD analysis of the extracts obtained from CCFD design was performed to confirm the results obtained by colorimetric methods and to identify the major phenolic compounds in the extracts. The HPLC analysis detected and confirmed the presence of six phenolic compounds in quinoa seed extracts in accordance with the results obtained by Repo-Carrasco-Valencia et al. (2010) for quinoa seeds. The peaks identified as free phenolic compounds corresponded to p-hydroxybenzoic, vanillic, p-coumaric and ferulic acids and the flavonoids quercetin and kaempferol. However, in comparison with the work published by Repo-Carrasco-Valencia et al. (2010), the presence of caffeic acid, myricetin and isorhamnetin was not detected.
For each studied operating condition, the sum of the amounts of individually phenolics determined by HPLC is given in last column of Table 1. The sum of the amounts of the four phenolic acids and the two flavonoids was higher in the extraction conditions considered as optimal by the model (60 °C; 80 % ethanol in the solvent and without ultrasounds), confirming once again the suitability of the employed model.
Conclusion
The results obtained after the analysis of response surfaces allowed optimizing the extraction conditions for antioxidant phenolic compounds from quinoa seeds. The optimum conditions of extraction temperature, ethanol content in the solvent and ultrasound power were determined for maximum extraction yields of TPC, TFC and higher antioxidant activity of the extracts. All responses varied considerably, principally as a function of the solvent composition. Therefore, ethanol content in the solvent is the key parameter on the extraction yields. The increase of ethanol content in the solvent and extraction temperature enhanced extraction of TPC, TFC and the antioxidant activity of the extracts. Ultrasound assistance was not appropriate for the extraction of antioxidant phenolic compounds from milled quinoa seeds under the experimental conditions used. Ultrasound assistance is not always appropriate to enhance the extraction yields and its efficiency is strongly related to the nature of the vegetal matrix, properties of the extracted compounds and the pretreatment of the vegetal source. The best extraction condition for maximum of all the evaluated responses was: extraction temperature of 60 °C, ethanol concentration in the solvent of 80 % and non-ultrasound assistance. Under these experimental conditions an antioxidant-rich extract was obtained, which exhibited a good in vitro antioxidant activity. In addition, the phenolic profile of this extract determined by HPLC-DAD allowed the identification of six free phenolic compounds. This study indicates that quinoa seeds can be considered a good source of naturally-occurring antioxidant compounds, which could have potential applications in food industry.
Acknowledgments
The authors would like to thank the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina) and Eurotango II (Erasmus Mundus programme) for the parcial support of this project (Ph.D. fellowships granted to Carciochi).
Conflict of interest
The authors declare that they have no conflicts of interest. This article does not contain any studies with human or animal subjects.
References
- Abugoch James LE. Quinoa (Chenopodium quinoa Willd.): composition, chemistry, nutritional, and functional properties. Adv Food Nutr Res. 2009;58:1–31. doi: 10.1016/S1043-4526(09)58001-1. [DOI] [PubMed] [Google Scholar]
- Alvarez-Jubete L, Wijngaard H, Arendt EK, Gallagher E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa, buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010;119:770–778. doi: 10.1016/j.foodchem.2009.07.032. [DOI] [Google Scholar]
- Bhargava A, Shukla S, Ohri D. Chenopodium quinoa—An Indian perspective. Ind Crop Prod. 2006;23:73–87. doi: 10.1016/j.indcrop.2005.04.002. [DOI] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Carciochi RA, Manrique GD, Dimitrov K. Changes in phenolic composition and antioxidant activity during germination of quinoa seeds (Chenopodium quinoa Willd.) Int Food Res J. 2014;21:767–773. [Google Scholar]
- Carrera C, Ruiz-Rodríguez A, Palma M, Barroso CG. Ultrasound assisted extraction of phenolic compounds from grapes. Anal Chim Acta. 2012;732:100–104. doi: 10.1016/j.aca.2011.11.032. [DOI] [PubMed] [Google Scholar]
- Galvan d’Alessandro L, Kriaa K, Nikov I, Dimitrov K. Ultrasound assisted extraction of polyphenols from black chokeberry. Sep Purif Technol. 2012;93:42–47. doi: 10.1016/j.seppur.2012.03.024. [DOI] [Google Scholar]
- Da Porto C, Porretto E, Decorti D. Comparison of ultrasound-assisted extraction with conventional extraction methods of oil and polyphenols from grape (Vitis vinifera L.) seeds. Ultrason Sonochem. 2013;20:1076–1080. doi: 10.1016/j.ultsonch.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Dini I, Tenore GC, Dini A. Antioxidant compound contents and antioxidant activity before and after cooking in sweet and bitter Chenopodium quinoa seeds. LWT Food Sci Technol. 2010;43:447–451. doi: 10.1016/j.lwt.2009.09.010. [DOI] [Google Scholar]
- Durling NE, Catchpole OJ, Grey JB, Webby RF, Mitchell KA, Foo LY, Perry NB. Extraction of phenolics and essential oil from dried sage (Salvia officinalis) using ethanol-water mixtures. Food Chem. 2007;101:1417–1424. doi: 10.1016/j.foodchem.2006.03.050. [DOI] [Google Scholar]
- Hamsaveni DR, Prapulla SG, Divakar S. Response surface methodological approach for the synthesis of isobutyl isobutyrate. Process Biochem. 2001;36:1103–1109. doi: 10.1016/S0032-9592(01)00142-X. [DOI] [Google Scholar]
- Hirose Y, Fujita T, Ishii T, Ueno N. Antioxidative properties and flavonoid composition of Chenopodium quinoa seeds cultivated in Japan. Food Chem. 2010;119:1300–1306. doi: 10.1016/j.foodchem.2009.09.008. [DOI] [Google Scholar]
- Koleva II, Van Beek TA, Linssen JPH, De Groot A, Evstatieva LN. Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem Anal. 2002;13:8–17. doi: 10.1002/pca.611. [DOI] [PubMed] [Google Scholar]
- Konishi Y, Hirano S, Tsuboi H, Wada M. Distribution of minerals in quinoa (Chenopodium quinoa Willd.) seeds. Biosci Biotechnol Biochem. 2004;68:231–234. doi: 10.1271/bbb.68.231. [DOI] [PubMed] [Google Scholar]
- Liyana-Pathirana C, Shahidi F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 2005;93:47–56. doi: 10.1016/j.foodchem.2004.08.050. [DOI] [Google Scholar]
- Ma YQ, Ye XQ, Fang ZX, Chen JC, Xu GH, Liu DH. Phenolic compounds and antioxidant activity of extracts from ultrasonic treatment of satsuma mandarin (Citrus unshiu Marc.) peels. J Agric Food Chem. 2008;56:5682–5690. doi: 10.1021/jf072474o. [DOI] [PubMed] [Google Scholar]
- Madhujith T, Shahidi F. Optimization of the extraction of antioxidative constituents of six barley cultivars and their antioxidant properties. J Agric Food Chem. 2006;54:8048–8057. doi: 10.1021/jf061558e. [DOI] [PubMed] [Google Scholar]
- Pingret D, Fabiano-Tixier AS, Chemat F. Degradation during application of ultrasound in food processing: a review. Food Control. 2013;31:593–606. doi: 10.1016/j.foodcont.2012.11.039. [DOI] [Google Scholar]
- Repo-Carrasco-Valencia R, Hellström JK, Pihlava JM, Mattila PH. Flavonoids and other phenolic compounds in Andean indigenous grains: Quinoa (Chenopodium quinoa), kañiwa (Chenopodium pallidicaule) and kiwicha (Amaranthus caudatus) Food Chem. 2010;120:128–133. doi: 10.1016/j.foodchem.2009.09.087. [DOI] [Google Scholar]
- Rice-Evans CA, Miller NJ, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. doi: 10.1016/S1360-1385(97)01018-2. [DOI] [Google Scholar]
- Silva EM, Rogez H, Larondelle Y. Optimization of extraction of phenolics from Inga edulis leaves using response surface methodology. Sep Purif Technol. 2007;55:381–387. doi: 10.1016/j.seppur.2007.01.008. [DOI] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1998;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Spigno G, De Faveri DM. Antioxidants from grape stalks and marc: Influence of extraction procedure on yield, purity and antioxidant power of the extracts. J Food Eng. 2007;78:793–801. doi: 10.1016/j.jfoodeng.2005.11.020. [DOI] [Google Scholar]
- Vega-Gálvez A, Miranda M, Vergara J, Uribe E, Puente L, Martínez EA. Nutrition facts and functional potential of quinoa (Chenopodium quinoa willd.), an ancient Andean grain: a review. J Sci Food Agric. 2010;90:2541–2547. doi: 10.1002/jsfa.4158. [DOI] [PubMed] [Google Scholar]