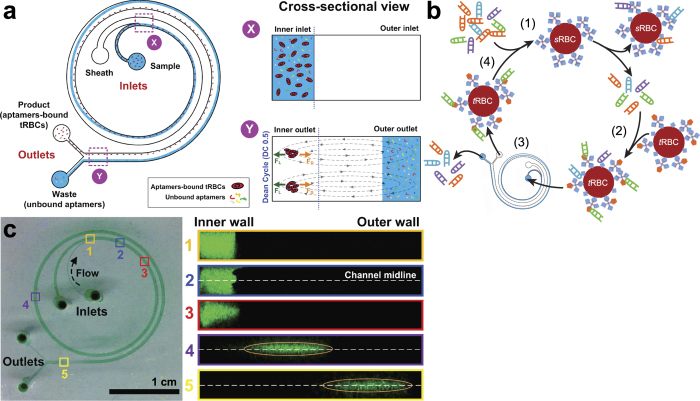
Figure 1. The I-SELEX microfluidic device and its use in aptamer selection is schematically illustrated.
(a) The microchannel design consists of a bi-loop spiral of radius ~1 cm with dual inlets and outlets. Pre-incubated bead/cell target-aptamer library mixtures and sheath buffer are pumped through the inner and outer inlets of the device, respectively. Under the influence of Dean drag forces (FD), unbound aptamers migrate along Dean vortices towards the outer wall and are diverted to the waste outer outlet. Target beads/cells (and any bound aptamers) experience additional strong inertial lift forces (FL) and are focused along the inner microchannel wall and collected in the product inner outlet. (b) A schematic of the I-SELEX procedure showing: (1) negative selection of random library against sRBCs; (2) positive selection of surviving library on tRBCs; (3) partitioning of tRBC-bound aptamers from unbound library in the I-SELEX device; (4) recovery, RT-PCR amplification and in vitro transcription to enrich tRBC-bound aptamers. (c) Optical cross sections through the device were taken at positions 1–5 along the length of the channel using high-speed confocal microscopy. Fluorescently labeled CRP aptamers injected via the sample inlet enter the microchannel nearest the inner wall (positions 1 and 2). Aptamers injected via the sample inlet are pinched tightly at the inner wall (position 1). Free aptamers begin to migrate across the channel as a tight band along the midline (positions 2-4) and exit the device when they reach the outer wall (position 5).