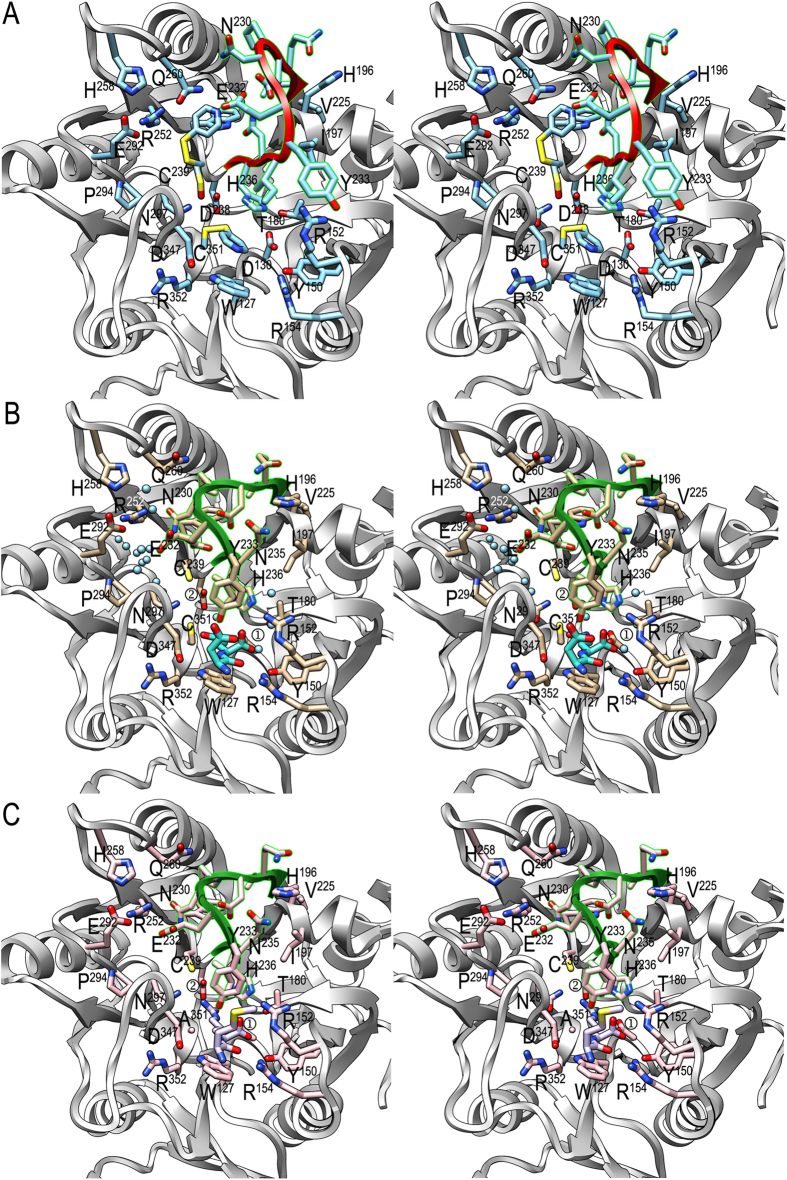
Figure 2. Active-site architecture.
(A) Stereo image of substrate-free PPAD CD, which actually corresponds to a thiopyridine modified state, with the Michaelis-loop (V226-V237) shown in red. Selected side chains are displayed with their carbons in light blue and labeled. (B) Same view as in (A) of the substrate-mimic complex, with the Michaelis-loop in green, unmodified cysteines, side-chain carbons in tan and relevant solvent molecules to illustrate the NH3-exit/H2O-entry and hydroxide channels as spheres in light blue (see also Fig. 4a). The bound aspartate-glutamine dipeptide is further shown with its carbons in turquoise. ① labels D130 and ② labels D238. (C) Same view as in (A) and (B) of the substrate complex, with the Michaelis-loop in green, unmodified cysteine C239 (C351 is replaced by alanine), and side-chain carbons in pink. The bound methionine-arginine dipeptide is further depicted with its carbons in purple. ① labels D130 and ② labels D238.