Fig. 1.
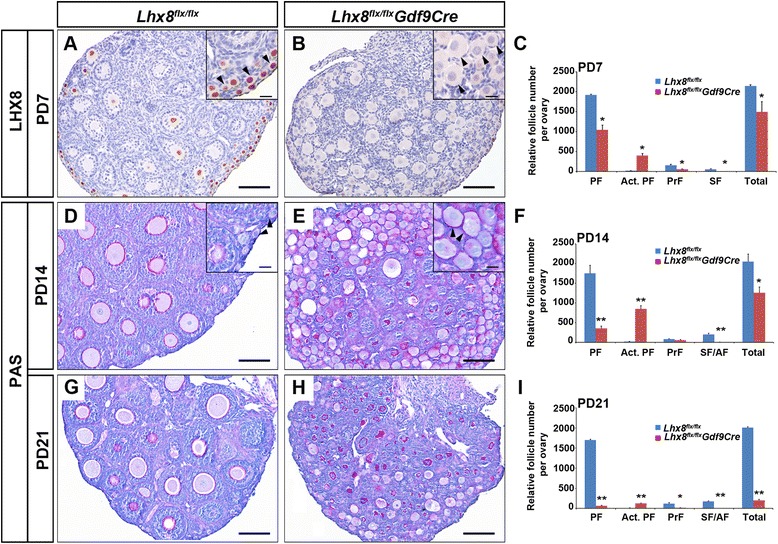
Postnatal inactivation of Lhx8 causes premature activation of primordial follicles and ovarian failure. a and b Anti-LHX8 antibodies were used to detect oocytes in paraformaldehyde-fixed and hematoxylin-counterstained ovaries taken at postnatal day 7 (PD7). Ovaries were derived from control (Lhx8 flx/flx, A) and Lhx8 conditional knockout (Lhx8 flx/flx Gdf9Cre, B) mice. Arrowheads in the inset of panel A indicate primordial follicles that stain with anti-LHX8 antibodies (brown signal). Arrowheads in the inset in panel B show activated primordial follicles (oocytes larger than 20 μm without cuboidal granulosa cells). d, e, g and h Periodic acid–Schiff (PAS) staining of PD14 (D and E) and PD21 (G and H) ovaries derived from control (D and G) and Lhx8 conditional knockout (E and H) mice. Arrowheads in the inset of panels D and E indicate primordial activated primordial follicles. c, f and i Quantification of ovarian follicle types in Lhx8 flx/flx and Lhx8 flx/flx Gdf9Cre mice. Five pairs of ovaries from PD7 (C), PD14 (F), and PD21 (I) were embedded in paraffin and serially sectioned at 5 μm thickness, and the follicles were counted. Anti-NOBOX antibodies stain oocyte nuclei throughout folliculogenesis and were used to identify oocytes in our counts. Every fifth section was counted. We scored primordial follicles (PF), activated primordial follicles (Act. PF, oocyte diameter greater than 20 μm without cuboidal granulosa cells), primary follicles (PrF) and secondary/antral follicles (SF/AF). *P < 0.05, **P < 0.01. Scale bars: 100 μm (A, B, D, E, G and H); 20 μm (inset in A, B, D and E)
