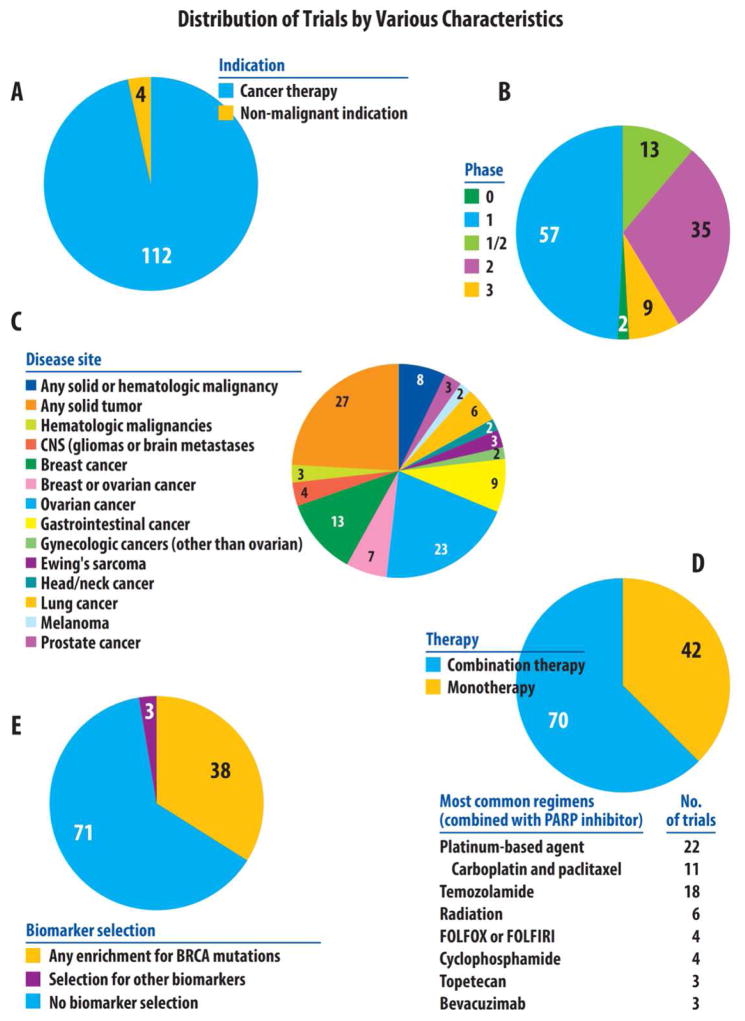
Figure 2.
Distribution of PARP inhibitor trials by various characteristics.
PARP inhibitor studies were identified on the clinical trial repository website (www.clinicaltrials.gov) using the keyword “PARP”. This search, completed in early March of 2015, identified 149 trials. Of these studies, 33 were excluded because they had been withdrawn prior to enrollment or because they did not utilize a true PARP inhibitor. Specifically, it should be noted that all studies including iniparib (BSI-201) were excluded, as it has been determined that this agent actually has poor selectivity toward PARP function, and does not meet the current criteria for a bona fide PARP1 inhibitor (Patel et al., 2012). Of the remaining 116 studies, the distribution of trials is shown by indication (cancer vs non-cancer indication) in Figure 2A and by phase (0–3) in Figure 2B. Of the 112 studies using PARP1 as cancer therapy, the distribution of these trials is then shown by disease site (Figure 2C), therapy (mono- vs combination therapy, Figure 2D), and by biomarker selection (Figure 2E).