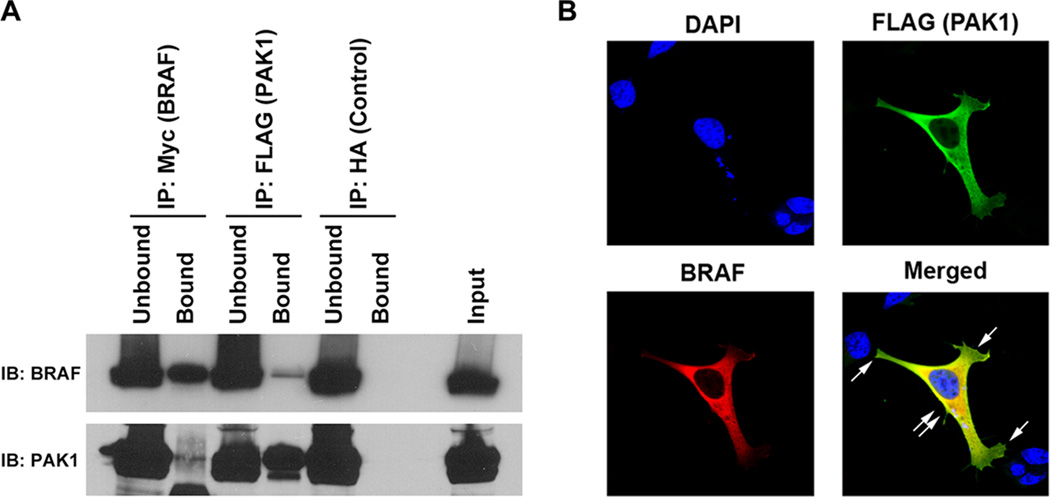
FIGURE 5.
Exogenous BRAF and PAK1 co-immunoprecipitate (IP) and co-localize. A. IP was performed on protein isolated from HEK293 cells that were transfected with cDNAs encoding Myc-tagged wild type BRAF and FLAG-tagged wild type PAK1. The IP using preconjugated anti-Myc and anti-FLAG antibodies demonstrated on Immunoblot (IB) using both the epitope tag and showed that the protein was precipitated and also that a small amount of BRAF precipitated with the FLAG IP and PAK precipitated with the Myc IP. Unbound is IB of the protein in the supernatant prior to washing and Bound is the immunoprecipitated protein. Input is IB of the total protein lysate serving as a size control. Non-specific binding was not identified using the protein precipitated with pre-conjugated anti-HA antibody (Control). B. Co-Transfected HEK293 cells were stained with DAPI (nuclear), Alexa-488 (FLAG) and Alexa-594 (BRAF) and subjected to immunofluorescence with confocal microscopy. Imaging revealed that BRAF and PAK1 co-localized (yellow, double arrow) in the perinuclear region, but not at the lamellopodia, which contained only PAK (single arrow). Data shown are representative of experiments performed on at least three occasions.