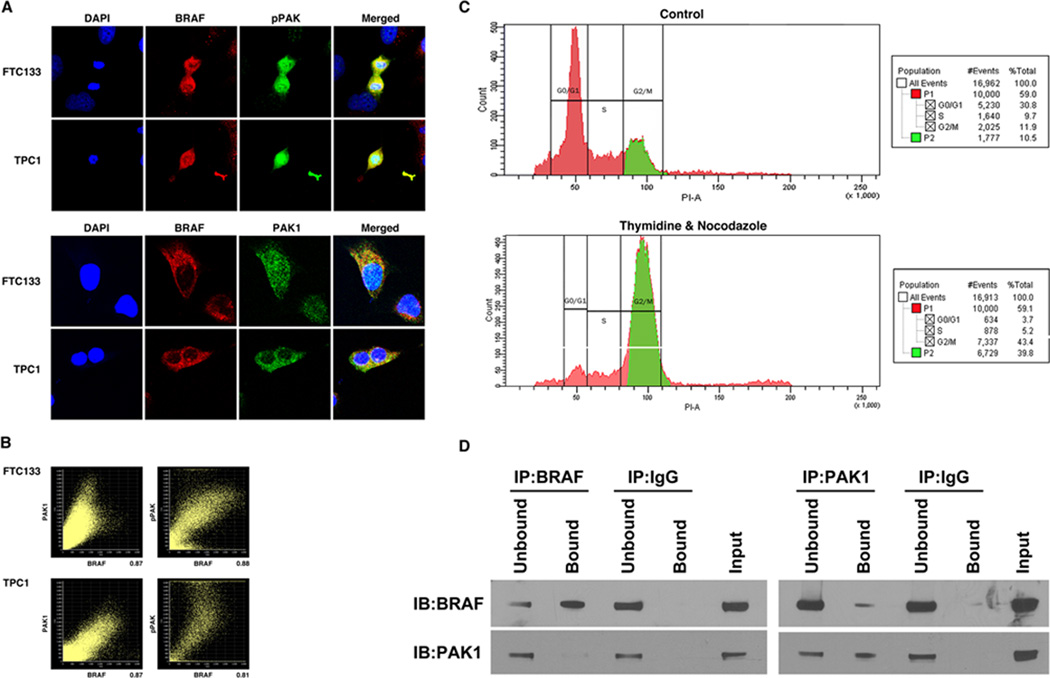
FIGURE 6.
Endogenous BRAF and PAK1 co-immunoprecipitate (IP) and co-localize A. TPC1 and FTC133 cells in continuous growth conditions were stained with DAPI (nuclear), Alexa-488 (PAK1& Thr423 pPAK) and Alexa-594 (BRAF) and subjected to immunofluorescence with confocal microscopy. Imaging revealed that BRAF co-localized with pPAK and PAK1. Low power and additional high power images are shown in Supplemental figure 1. B. In representative FTC133 cells, Person’s coefficient for co-localization of BRAF and PAK1, and BRAF and pPAK were 0.74496 and 0.86779 with percentages of 87% and 88%, respectively. In representative TPC1 cells, Person’s coefficient for co-localization of BRAF and PAK1, and BRAF and pPAK, were 0.83179 and 0.75334 with percentages of 87% and 80%, respectively. C. TPC1 cells were treated with thymidine and nocodazole, which increased the percentage of cells in G2/M phase (39.8%) compared to the untreated control (10.5%). D. Protein was isolated from thymidine and nocodazole treated TPC1 cells and IP was performed using BRAF and PAK1 antibodies, and IgG as a negative control. IB of the precipitated protein demonstrated that BRAF-precipitated protein contained PAK1 and that PAK1-precipitated protein contained BRAF with no non-specific binding with IgG. Unbound is IB of the protein in the supernatant prior to washing and Bound is the immunoprecipitated protein. Input is IB of the total protein lysate to confirm size. Data shown are representative of experiments performed on at least two occasions.