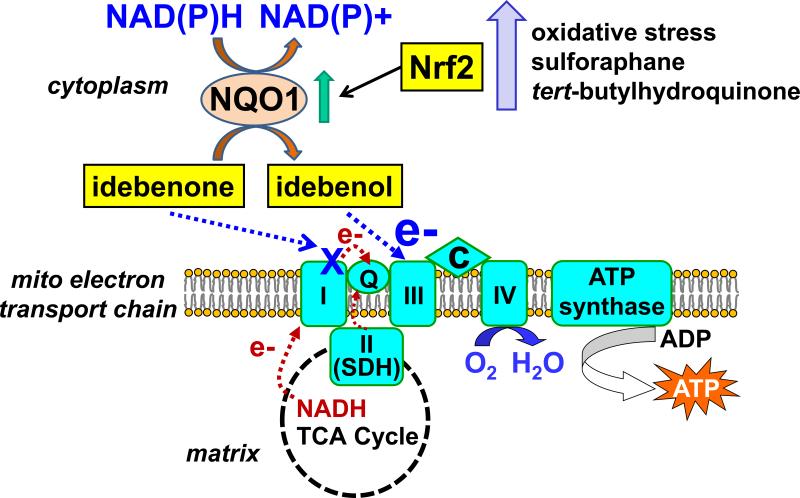
Fig. 3.
Model of NQO1-dependent shuttling of reducing equivalents from cytoplasmic NAD(P)H to mitochondrial electron transport chain complex III via idebenone. The two-electron reduction of idebenone to idebenol is catalyzed by NAD(P)H:quinone oxidoreductase 1 (NQO1) and occurs primarily in the cytoplasm. Idebenol is hydrophilic enough to traverse the cytoplasm but lipophilic enough to mediate electron transfer to complex III in the mitochondrial inner membrane. NQO1-catalyzed idebenone reduction may elude the deleterious consequences of idebenone-complex I interaction while idebenol may exert cytoprotection by bypassing disease-associated inhibition of complex I or upstream tricarboxylic acid cycle (TCA) enzymes. This bypass would restore oxygen (O2) consumption at complex IV and ATP production by the ATP synthase. Stimuli which activate the nuclear factor erythroid 2-related factor 2 (Nrf2) transcription factor, including mild oxidative stress and the drugs sulforaphane and tert-butylhydroquinone, are predicted to synergize with idebenone by upregulating NQO1 expression