Fig. 1.
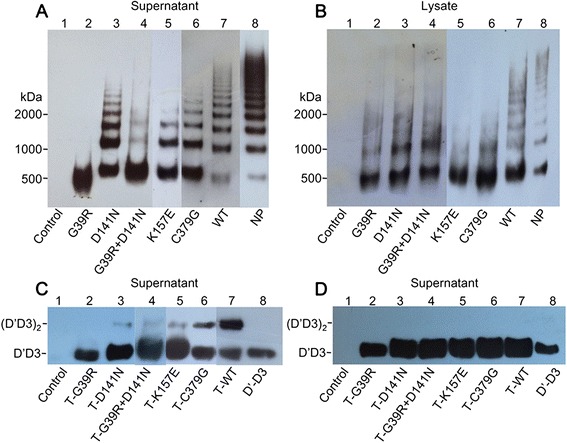
Defective multimerization of mutant VWF and reduced dimerization of mutant truncated VWF. a HEK293 cells were transiently transfected by equal WT or mutant full-length VWF plasmid. Seventy hours after transfection, the supernatant of cells were collected for multimer analysis. In order to detect whether the mutations have abnormal multimer pattern, we measured equal VWF antigen of the mutations, wild type, and NP. Lane 1 is the negative control of the mimic empty vector. Lanes 2–6 show the supernatant obtained from VWF mutant transfections and indicate decreases in high to medium-sized multimers to varying degrees. Exclusively, dimers formed with the Gly39Arg mutant (lane 2). The ability to form multimers was partly recovered by co-transfection of Gly39Arg and Asp141Asn (lane 4). b In the lysates of transfected cells, a similar decrease in the formation of VWF multimers was seen in the variants. However, WT-VWF exhibited a multimer pattern as observed for normal plasma. c HEK293 cells were transiently transfected by equal WT or mutant truncated VWF. In the media of transfected cells, dimerizations of truncated recombinant VWF were detected by Western blotting under the non-reducing condition. Lane 1 shows the empty pSecTag2/Hygro B vector. Dimers were absent in the Gly39Arg mutant (lane 2) while lanes 3–6 tapered in the dimer to different degrees. d In the supernatant of the transfected cells, the truncated VWF mutations and WT D1D2D′D3 fragment were examined under the reducing condition, and only D′D3 monomers were found
