Figure 3. Detection of CHD4– and CHD5–NuRD components: GST–FOG1 pull-down.
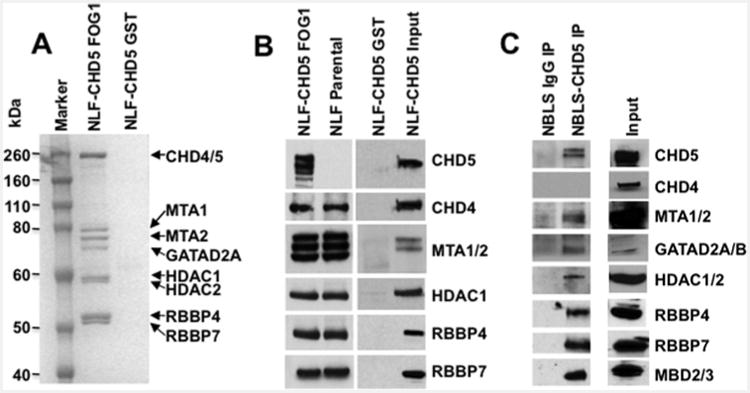
(A) Nuclear extracts of NLF–CHD5 were subjected to either GST alone or GST–FOG1 pull-down followed by PAGE and stained with Simply Blue. All canonical NuRD components from CHD4/5–NuRD complexes were readily visible (marked with arrows). (B) GST–FOG1 bound protein complexes from NLF–CHD5 and NLF parental cells were subjected to 4%–12% PAGE. The identity of individual proteins from the NuRD complex was confirmed by Western blot analysis. CHD5 was detected only in NLF–CHD5-S extracts but absent from NLF extracts. Additional bands in the CHD5 lane are probably due to proteolytic degradation products and additional bands with MTA1/2 probably represent alternate isoform recognition. (C) Identification of individual canonical NuRD components in NBLS cells. Nuclear extracts were immunoprecipitated with either IgG or CHD5 antibody as indicated and complexes were subjected to SDS/PAGE (4%–12% gels) followed by Western blot analysis. MTA1/2, GATAD2A/B, HDAC1/2, RBBP4/7 and MBD2/3 were detected as individual canonical NuRD components along with CHD5.
